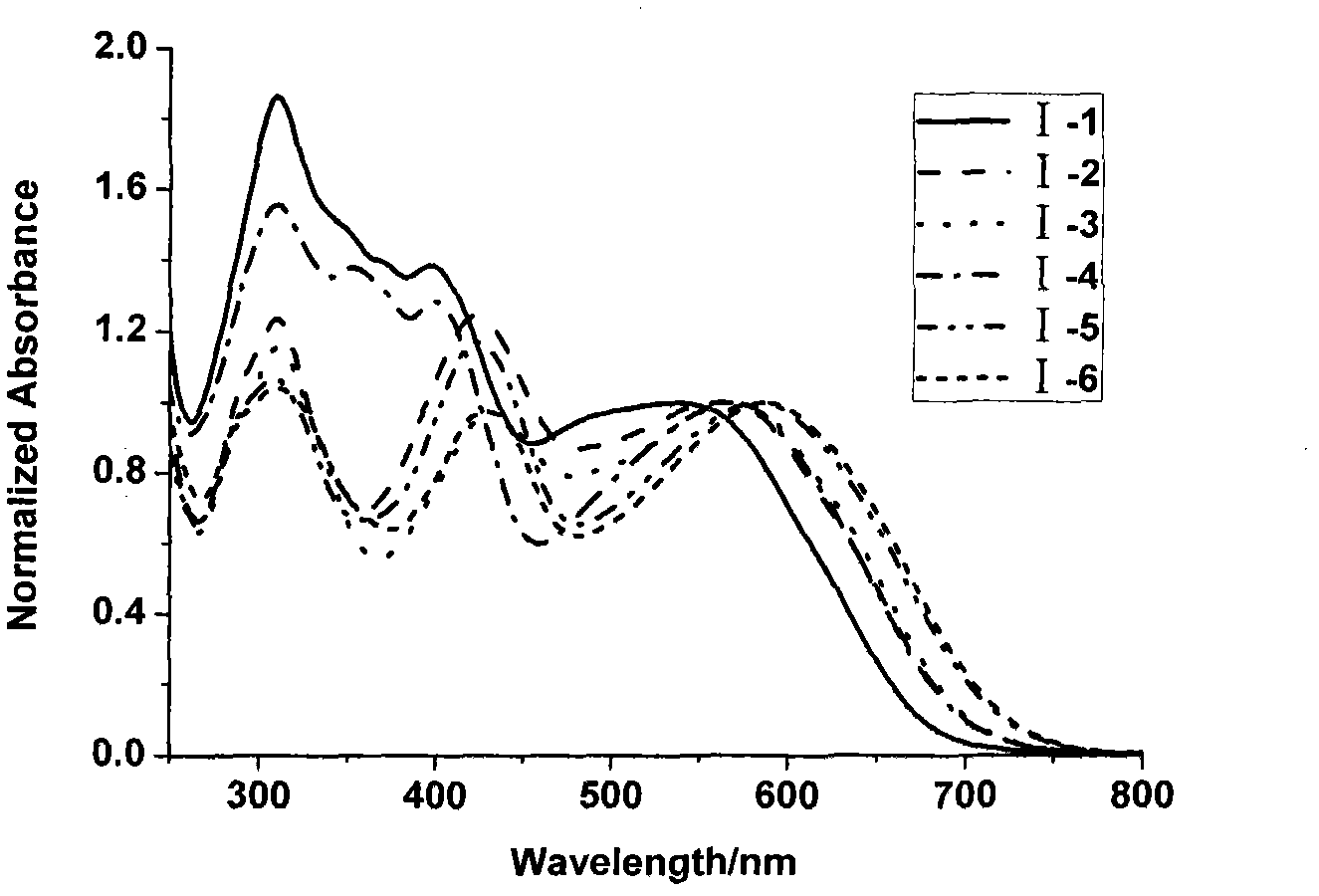
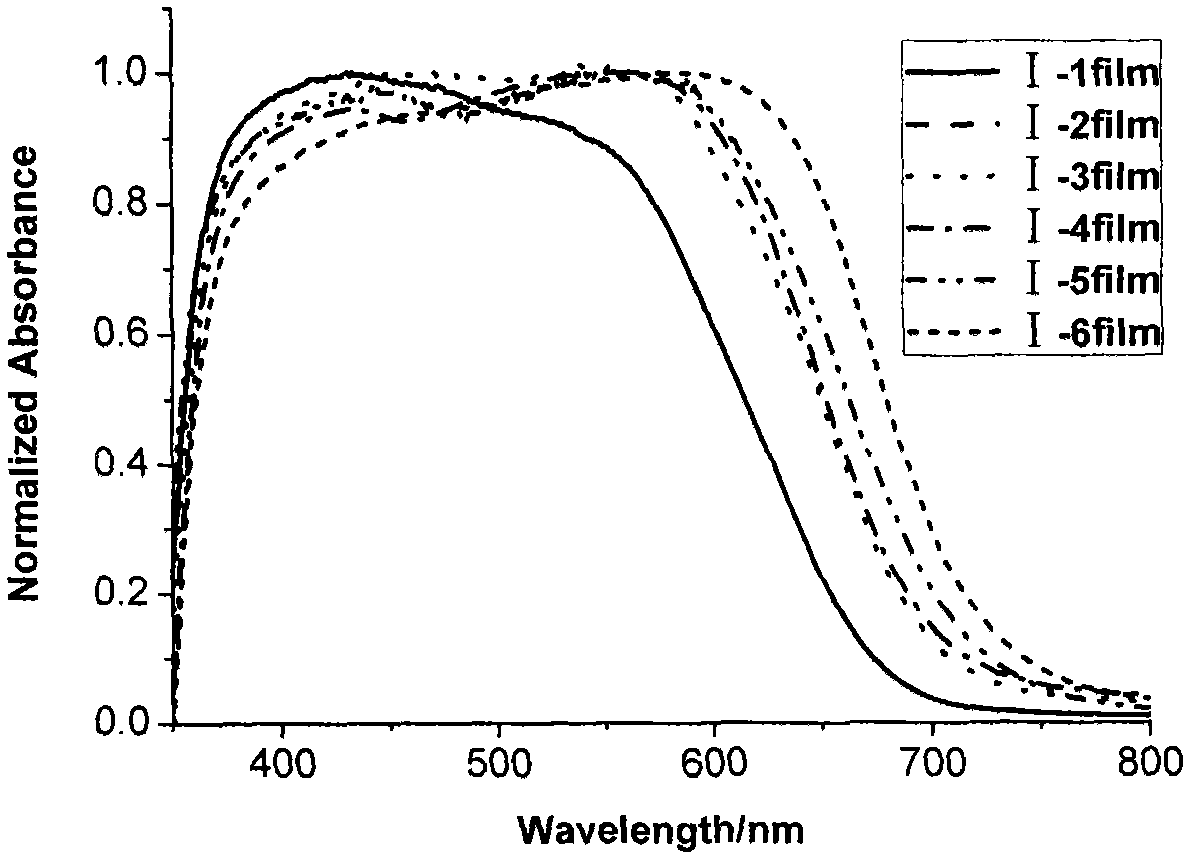
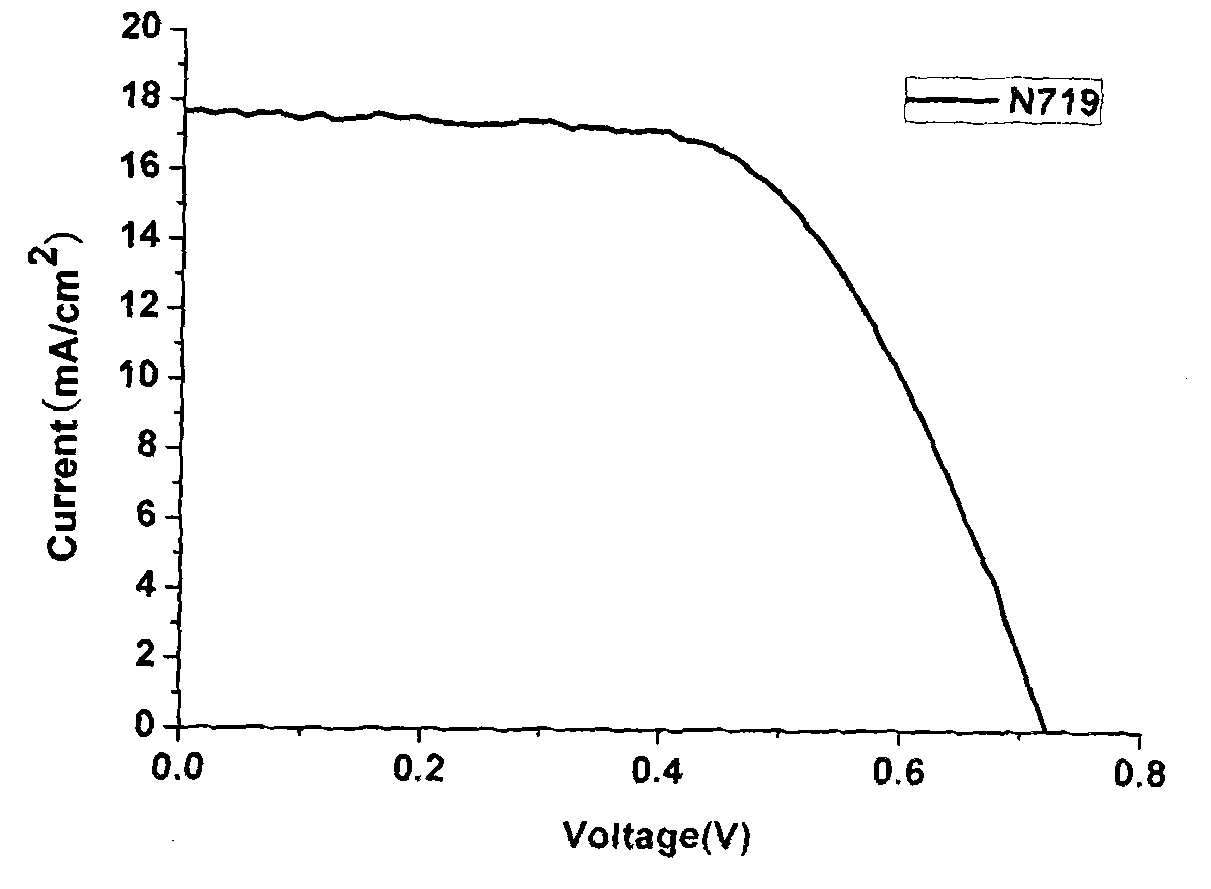
Iso-indigo derivative and applications of the iso-indigo derivative
A derivative, isoindigo technology, which is used in bis-indo-indocyanine indigo dyes, electrolytic capacitors, photovoltaic power generation, etc., can solve the problems of high price and complicated preparation process, and achieve low cost, easy preparation and cost reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The method for preparing target compound (compound shown in I formula) provided by the present invention, its main synthetic route is as follows:
[0037]
[0038] Among them: Ar 1 , Ar 2 , R 1 and R 2 has the same meaning as described above. The method comprises the steps of:
[0039] (1) In alkaline and catalyst [such as Pd 2 (dba) 3 etc.] and the presence of inert gas, compound A and (HO) 2 B-Ar 2 -CHO was placed in an aprotic polar solvent [such as tetrahydrofuran (THF), etc.], and kept at reflux for at least 10 hours, cooled, evaporated to remove the solvent, and the residue was dissolved in dichloromethane, washed with water, and the dichloromethane layer was washed with no dried over sodium sulfate, spin-dried, and subjected to silica gel column chromatography to obtain compound B;
[0040] (2) Also in alkaline and catalyst [such as Pd 2 (dba) 3 etc.] and the presence of inert gas, compound B and Ar 1 B(OH) 2 Repeat step (1) to obtain compound C; ...
Embodiment 1
[0059]
[0060] Add 533mg (2.36mmol) of compound 1, 500mg (2.36mmol) of compound 2, 15mL of glacial acetic acid and 0.1mL of concentrated hydrochloric acid into a 25mL two-neck round bottom flask, and heat to reflux for 24 hours. After cooling and suction filtration, the solid was washed with water, ethanol and ethyl acetate respectively, and vacuum-dried to obtain a brown solid (compound 3), with a yield of 90%.
[0061] 1 H NMR ((CD 3 ) 2 NCOD), δ: 10.7 (bs, 2H), 9.14 (d, J=8.7Hz, 2H), 7.22-7.15 (m, 4H). 1.35-1.15 (m, 6H), 0.86 (t, 3H). 13 C NMR (CDCl 3 ), δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
[0062]
[0063] 420 mg (1 mmol) of compound 3 and 824 mg (6 mmol) of potassium carbonate were added to a 50 mL two-neck round bottom flask under argon protection, and 0.384 mL (2.2 mmol) of 1-bromooctane was injected with a syringe, and reacted at 100° C. for 18 hours. After cooling, it was poured into 200ml of water, stirred for 2 hours, and a gray-re...
Embodiment 2
[0077]
[0078] In a 50mL dry flask, add 322mg (0.5mmol) A-1, 15mg Pd 2 (dba) 3 , 10mg P(o-tyl) 3 and 0.5g of potassium phosphate, under the protection of argon, inject 12mL of tetrahydrofuran with a syringe. Heat to 70°C, inject 98mg (0.7mmol) of furanboronic acid aldehyde dissolved in 10mL tetrahydrofuran, raise the temperature to 85°C after the addition, and stir for 20h. Cool to room temperature, spin off the solvent, dissolve the residue with dichloromethane, wash with water, dry the dichloromethane layer with anhydrous sodium sulfate, spin dry, perform silica gel column chromatography, the developing solvent is petroleum ether / dichloromethane=2 / 1 To 1 / 1 (V / V), 168 mg of brown solid (compound B-2) was obtained, yield 51%.
[0079] 1 H NMR (CDCl 3 , 400MHz), δ: 9.70(s, 1H), 9.25(d, J=8Hz, 1H), 9.08(d, J=8Hz, 1H), 7.43(dd, J 1 =8Hz,J 2 =8Hz, 1H), 7.37(d, J=4Hz, 1H), 7.22(s, 1H), 7.18(dd, J 1 =12Hz,J 2 =8Hz, 1H), 6.98(d, J=4Hz, 1H), 6.93(s, 1H), 3.84(t, 2H), 3.74...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com