Method for producing L-ornithine hydrochloride by genetic engineering bacteria
A technology of ornithine hydrochloride and genetically engineered bacteria, which is applied in the field of production of L-ornithine hydrochloride and can solve problems such as difficulty in filtration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
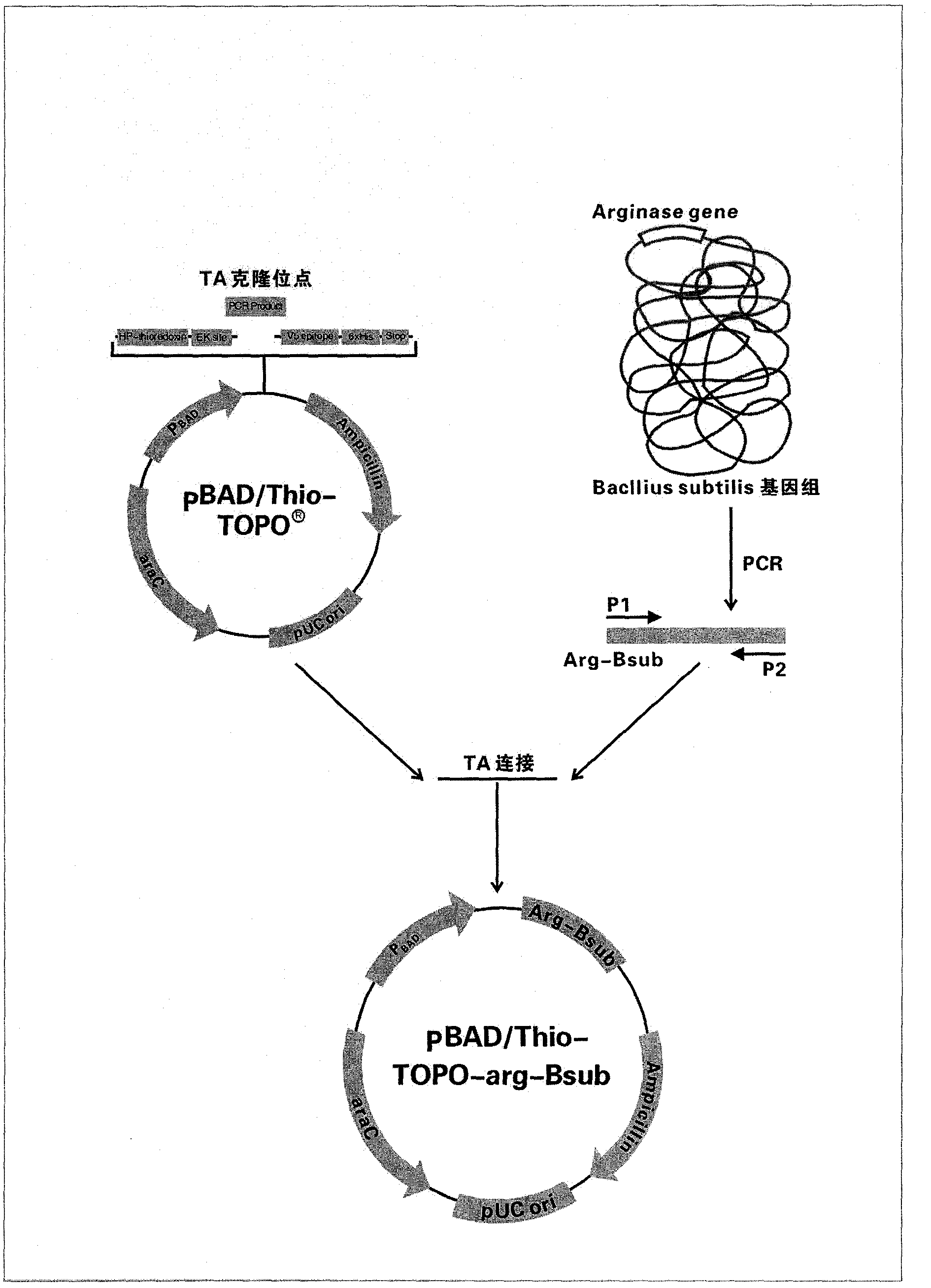
[0181] Design and synthesize a pair of TA cloning primers
[0182] Log in to GenBanK and obtain the arginase gene of Bacillus Subtilis 168 strain through Gene ID: 937760 ( arg The complete coding sequence of Bsub), using Primerer 5.0 software to design a pair of PCR primers, p1, p2, for amplifying the DNA sequence of Bacillus subtilis arginase gene, and performing TA ligation with pBAD / Thio-TOPO vector. Primers were synthesized by Yingwei Jieji (Shanghai) Trading Co., Ltd.
[0183] P1: 5′-GAAATGGATAAAACGATTTCGGTTAT-3′, a total of 26 bp.
[0184] P2: 5′-GGTCAGCAGCTTCTTCCCTAACA-3′, a total of 23 bp.
Embodiment 2
[0186] Extraction and determination of Bacillus subtilis genomic DNA
[0187] Genomic DNA of Bacillus subtilis was extracted by the improved method of Palva I et al.
[0188] (1). Pick a single bacterium colony cultured on the plate of Bacillus subtilis (preservation number CCTCC No. 93009), inoculate it into a test tube with 10 ml of beef extract and peptone liquid medium, and culture it with shaking at 32° C. for 14 hours.
[0189] (2). Centrifuge at 5000rpm for 10min to obtain bacterial pellet, wash once with STE, centrifuge again, and resuspend the bacterial cell in 4ml TE solution.
[0190] (3). Add 8 μl of 50 mg / ml lysozyme solution (final concentration is 100 μg / ml), and incubate at 37°C for 20 minutes. Then add RNase (10mg / ml) 10μl to a final concentration of 25μg / ml, then add 10% SDS to dissolve 0.5ml, and keep warm at 37℃ for 30min. Add proteinase K (20mg / ml) 10μl, the final concentration is 50μg / ml, keep at 37℃ for 60min.
[0191] (4). Then add an equal volume of...
Embodiment 3
[0200] PCR Amplification of Arginase Gene of Bacillus subtilis
[0201] 1). Prepare 50 μl reaction system.
[0202] Template DNA, 2μl (50ng); 10 X PCR Buf, 5μl; 50mMdNTPs, 0.5μl;
[0203] Primers p1 and p2, each 1μl (100ng); Taq DNA polymerase (1 unit / μl), 1μl; add sterile pure water to a volume of 50μl.
[0204] The program of PCR reaction was: pre-denaturation at 94°C for 4min, denaturation at 94°C for 1min, annealing at 56°C for 30sec, extension at 72°C for 1.5min; a total of 30 cycles, and finally extension at 72°C for 10min. After the reaction was completed, temporarily store in ice until use.
[0205] 2). Take 2μl of the amplified product and load it on 1.2% agarose gel electrophoresis to detect a 0.9KB DNA fragment. The DNA band has a clear outline and is suitable for direct TOPO-TA cloning reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com