Preparation method of 2-(4-alkylphenyl) propanoic acid
A technology of alkylphenyl and alkylbenzene, applied in the field of synthesis of 2-propionic acid, achieving the effects of low production cost, low reaction conditions, and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0026] The preparation of embodiment 12-ethyl chloropropionate
[0027] Mix 10.9 g of 2-chloropropionic acid, 10 ml of absolute ethanol and 0.2 g of 98% concentrated sulfuric acid, add 10 ml of toluene to carry water, and reflux for 2 hours under a water bath condition of 80-100 ° C. Add 20% sodium hydroxide solution to the reacted mixed solution to adjust the pH value to 9, separate with a separatory funnel to obtain a toluene layer, dry the product with anhydrous sodium sulfate, and rotary evaporate to obtain ethyl 2-chloropropionate.
Embodiment 22
[0028] The synthesis of embodiment 22-(p-methylphenyl) ethyl propionate
[0029] Place 0.1mol of ethyl 2-chloropropionate, 60ml of toluene and 2 grams of anhydrous aluminum chloride in a three-necked flask, and a low-temperature circulation pump (using absolute ethanol as a refrigerant in laboratory conditions) controls the temperature at 0°C, mechanically The reaction was stirred for 24 hours. Friedel-Crafts reaction of ethyl 2-chloropropionate with toluene produces ethyl 2-(p-methylphenyl)propionate.
Embodiment 32
[0030] The preparation of embodiment 32-(p-methylphenyl) propionic acid
[0031] Add 10% dilute hydrochloric acid to the product of Example 2 and heat to 80° C. to hydrolyze the ester, and recover ethanol by distillation. After 20 minutes of reaction, add water to destroy the unfinished aluminum chloride. The toluene layer was obtained by liquid separation, and the obtained mixture was evaporated to toluene to obtain the product 2-(p-methylphenyl)propionic acid.
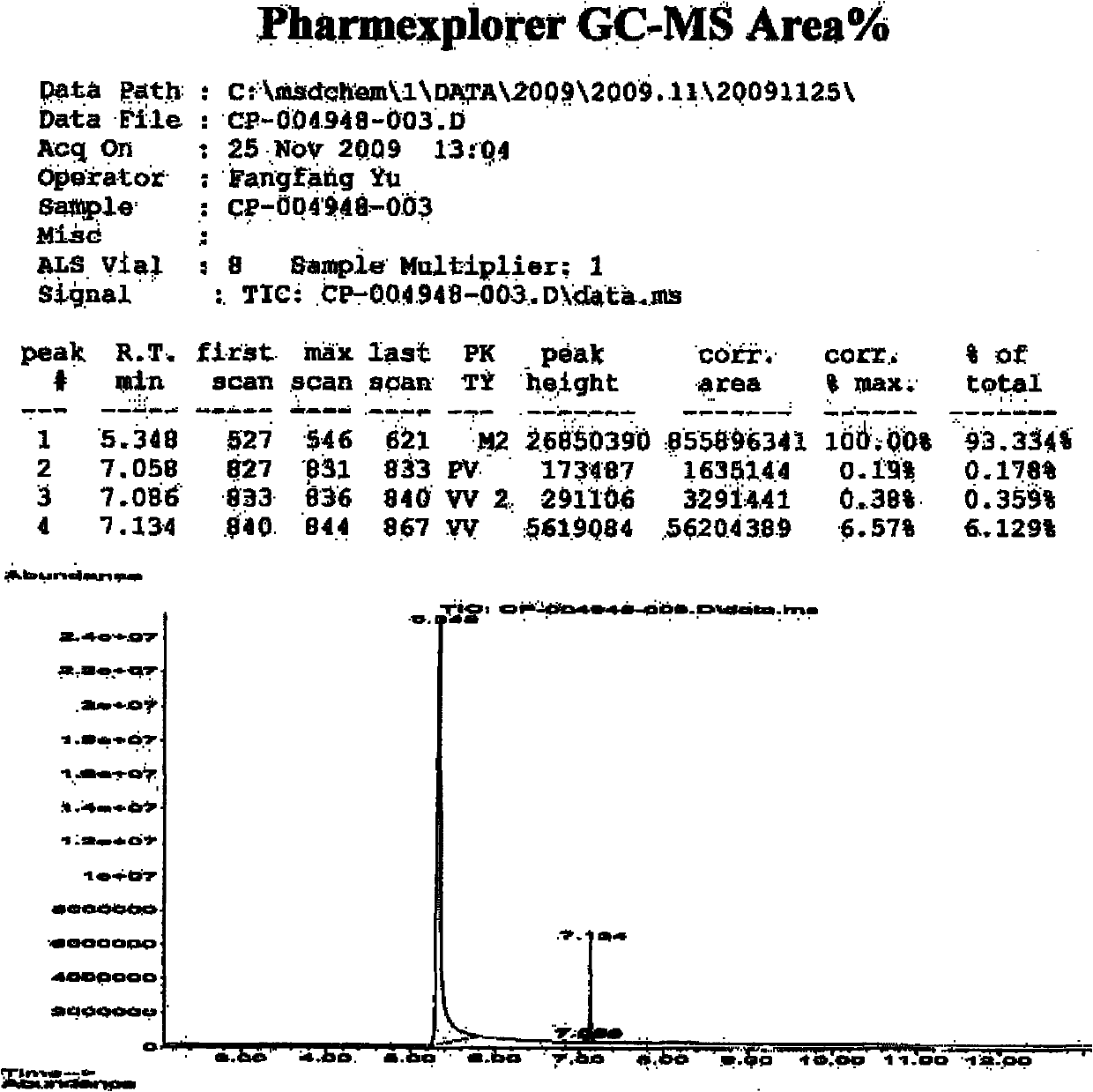
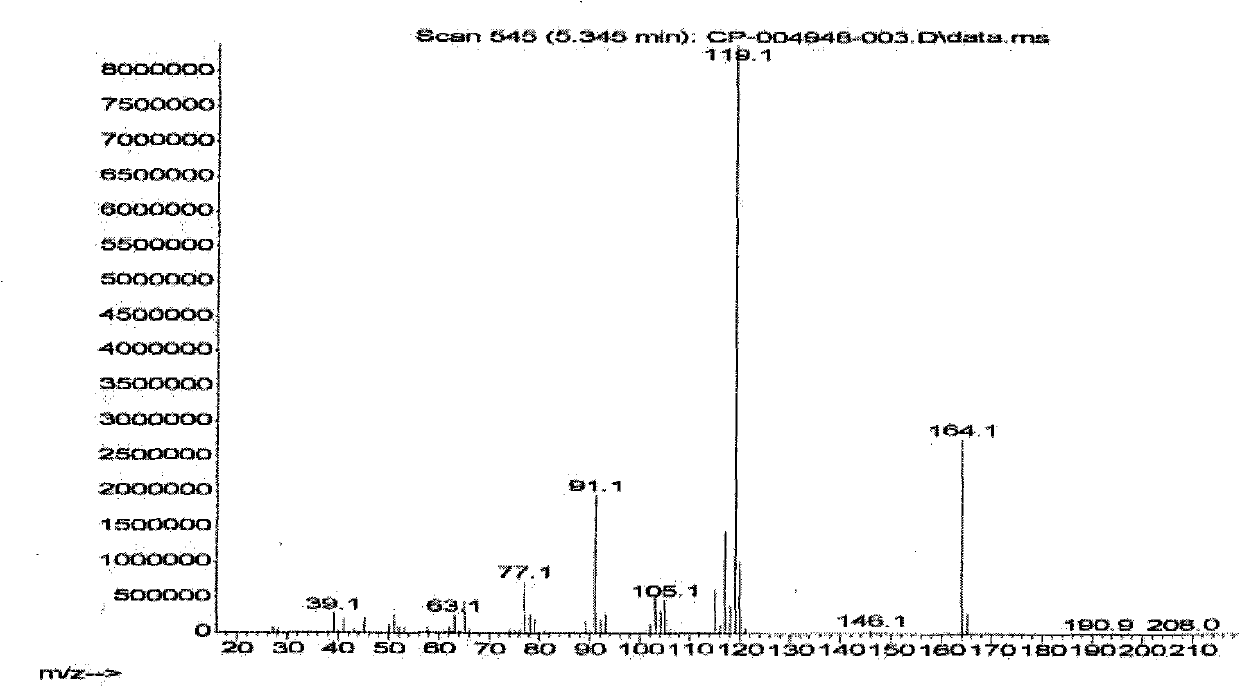
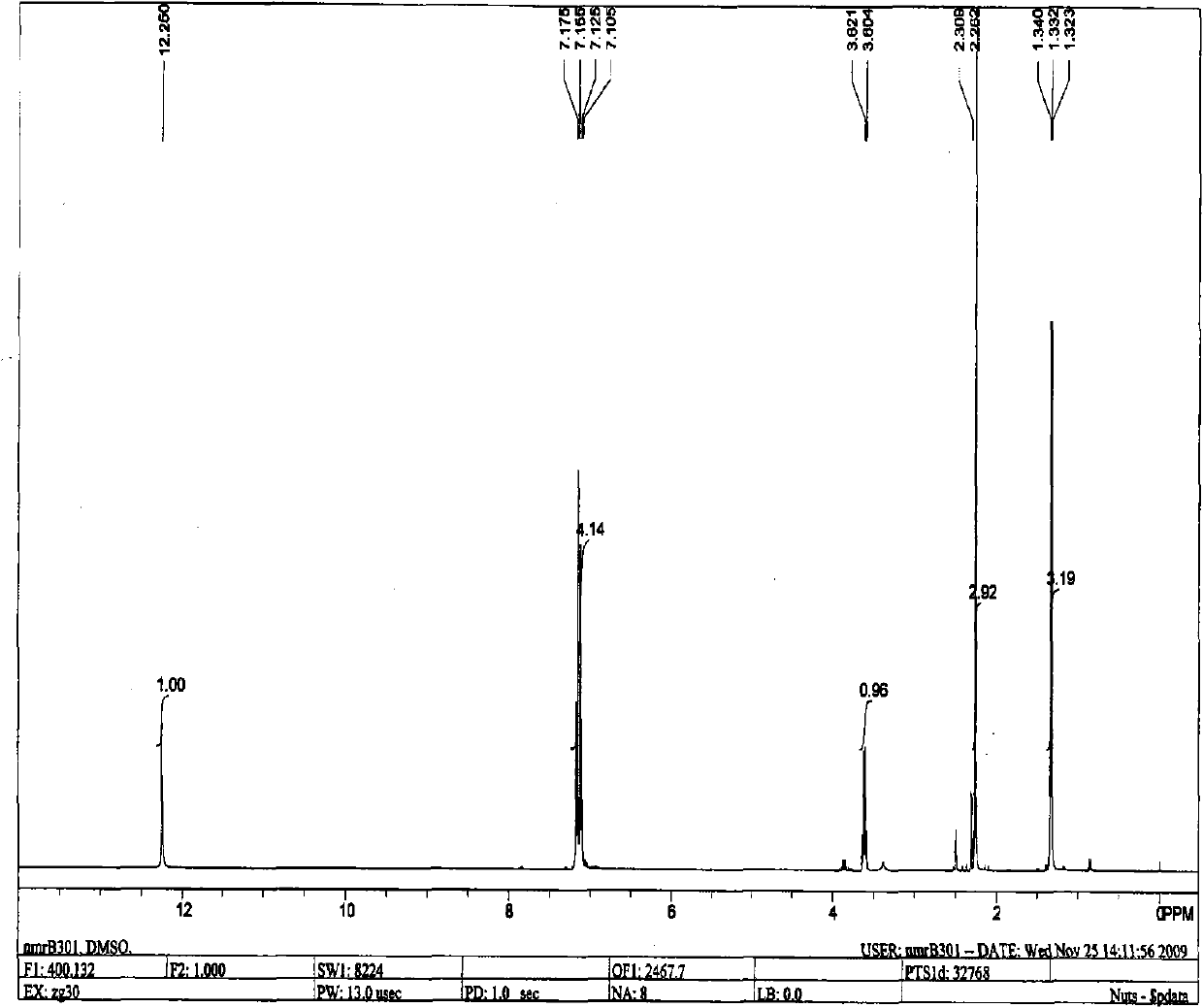
[0032] The prepared sample was submitted for inspection, and the relative molecular mass of the determined sample was 164.1, which was consistent with the relative molecular mass of 2-(p-methylphenyl)propionic acid. The GCMS spectrum is shown in (a) and (b) of Figure 1, and the hydrogen nuclear magnetic resonance spectrum ( figure 2 ) shows that there are 5 groups of peaks, the integral values are 1.00, 4.14, 0.96, 2.92, 3.19 respectively, and the ratio is close to 1:4:1:3:3, which is consistent with the molecula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com