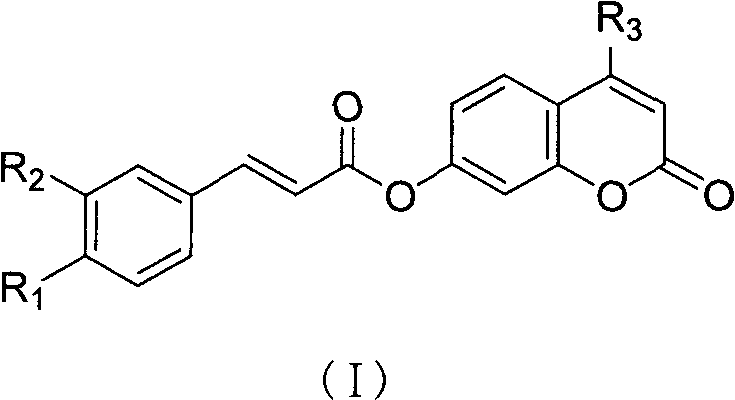
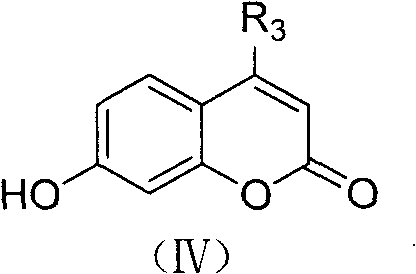
Method for preparing caffeic acid hydroxycoumarin ester compounds
A technology of ester compound and hydroxycoumarin, which is applied in the formation/introduction of carboxylate groups, skin care preparations, cosmetics, etc., can solve the problems of cumbersome operation, high cost, and influence on caffeic acid ester, and achieve shortening Effects of reaction time, simplified operation, and reduced reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of E-3-(3,4-dihydroxyphenyl)-2-acrylic acid-4-methylumbelliferone ester
[0031] (1) Add 0.5 g (3 mmol) of caffeic acid into a three-neck flask, add 10 ml of thionyl chloride to reflux for 2 hours, extract the thionyl chloride under reduced pressure, dissolve the residue in 5 ml of xylene, and transfer it to the dropping funnel spare.
[0032] (2) Grind 1.1g (6mmol) of 4-methylumbelliferone in a pulverizer, suspend in 4ml xylene, and pass into N 2 Gas, heated to boiling (reaction temperature is about 137 ~ 140 ° C), dropwise added the solution obtained in the above (1) step under stirring, controlled the rate of addition, slowly added dropwise, the dropwise addition was completed in about 1h40min, and continued to react for 15-20min (A total of about 2 hours of reaction), the reaction was stopped, cooled, filtered, washed with ether, washed with water, dried, and recrystallized with ethanol to obtain 0.65 g of light yellow flaky crystals, yield: 69.2%, mp22...
Embodiment 2
[0034] Preparation of E-3-(3,4-dihydroxyphenyl)-2-acrylic umbelliferyl ester
[0035] The preparation method and conditions are the same as in Example 1.
[0036] Raw material charging ratio: caffeic acid 0.5g (3mmol), thionyl chloride 10ml, umbelliferone 1g (6mmol), xylene consumption is the same as Example 1, recrystallizes with ethanol to obtain light yellow needle crystal 0.62g, yield: 68.9%, mp 227.8°C (Dec). Elemental analysis, found value (calculated value), %: C 66.79 (66.67), H 3.81 (3.73). 1 HNMR (DMSO-d6), δ: 6.45~6.47 (d, 1H, pyran-=CHCO), 6.49~6.52 (d, 1H, =CHCO), 6.79~7.34 (m, 5H, Ph), 7.70~7.73 (d, 1H, pyran-Ph CH=), 7.76~7.78(d, 1H, ph), 8.06~8.08(d, 1H, Ph-CH=), 9.28(S, 1H, OH), 9.72(S , 1H, OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com