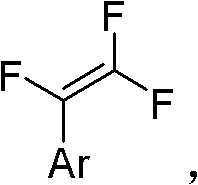
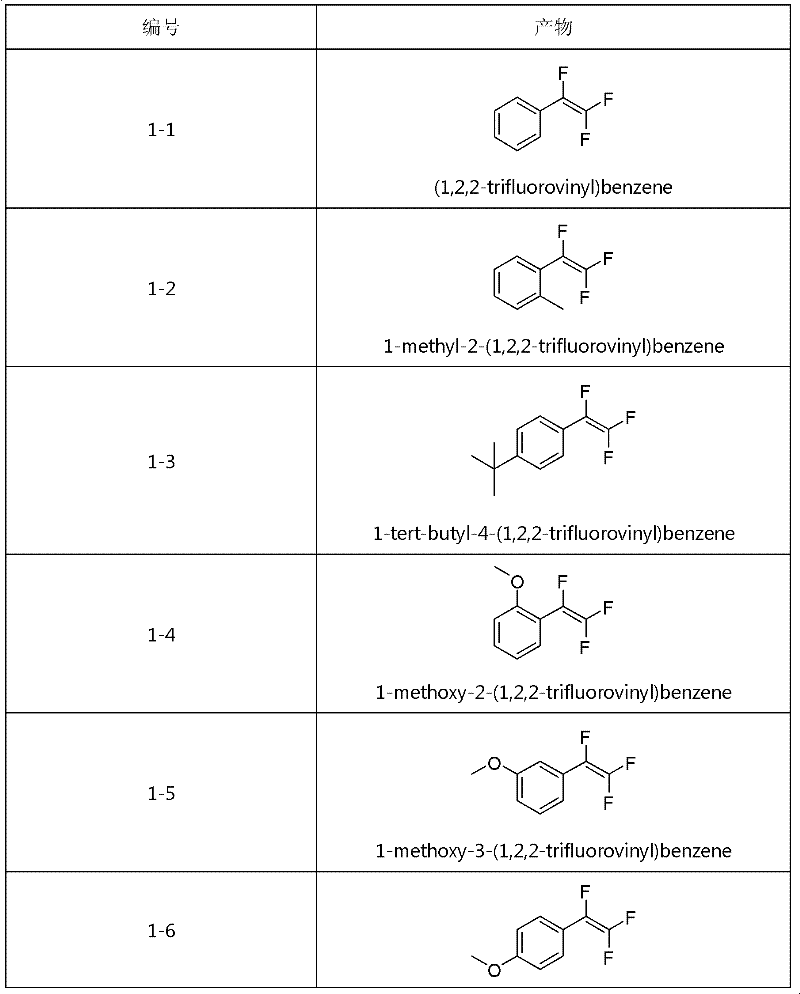
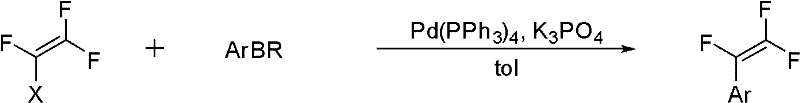Method for synthesizing trifluorostyrene fluorine-containing monomer
A technology of trifluorostyrene and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of low selectivity, low price, low yield, etc., and achieve easy availability of raw materials. , the effect of the simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of 1,2,2-trifluorostyrene
[0030] Add phenylboronic acid (122mg), tetrakis(triphenyl)phosphine palladium (11.5mg), potassium phosphate (636mg) into the reaction tube with external condensing reflux jacket and branch pipe (without feeding sequence), add 2mL toluene solvent and appropriate amount After water, a dry ice reflux device was connected to the reaction tube to exchange argon. Fill the balloon with 5 times equivalent of chlorotrifluoroethylene and connect it to the branch tube of the reaction tube, then turn on the water in the outer condenser tube and then raise the temperature of the reaction tube to 95°C. After adding the dry ice acetone bath, open the branch valve, put in chlorotrifluoroethylene, keep the dry ice refluxed for 6 hours, and then pass the column with n-hexane to obtain a colorless liquid with a yield of 78%. 1 H NMR(300MHz, CDCl 3 ,293K,TMS)δ7.54-7.38(m,5H)ppm; 19 F NMR(282MHz, CDCl 3 , 293K, TMS)δ-100.12(dd, J=54.1, 25.1Hz, 1F...
Embodiment 2
[0031] Example 2 Synthesis of 1-methyl-2-(1,2,2-trifluorovinyl)benzene
[0032] Add o-toluene boronic acid (136mg), tetrakis(triphenyl)phosphine palladium (11.5mg), potassium phosphate (636mg) into the reaction tube with external reflux jacket and branch pipe (without feeding order), and add 2mL toluene After solvent and appropriate amount of water, a dry ice reflux device was connected to the reaction tube to exchange argon. Fill the balloon with 5 times equivalent of chlorotrifluoroethylene and connect it to the branch tube of the reaction tube, then turn on the water in the outer condenser tube and then raise the temperature of the reaction tube to 95°C. After adding the dry ice acetone bath, open the branch valve, put in chlorotrifluoroethylene, keep the dry ice refluxed for 6 hours, and then pass the column with n-hexane to obtain a colorless liquid with a yield of 77%. 1 H NMR(300MHz, CDCl 3 , 293K, TMS) δ 7.38-7.32 (m, 3H), 7.23-7.20 (m, 1H), 2.43 (s, 3H) ppm; 19 F NMR(282...
Embodiment 3
[0033] Example 3 Synthesis of 1-tert-butyl-4-(1,2,2-trifluorovinyl)benzene
[0034] Add 4-tert-butylphenylboronic acid (178mg), tetrakis(triphenyl)phosphine palladium (11.5mg), potassium phosphate (636mg) into the reaction tube with external reflux jacket and branch pipe (without feeding order), add After 2 mL of toluene solvent and an appropriate amount of water, a dry ice reflux device was connected to the reaction tube to exchange argon. Fill the balloon with 5 times equivalent of chlorotrifluoroethylene and connect it to the branch tube of the reaction tube, then turn on the water in the outer condenser tube and then raise the temperature of the reaction tube to 95°C. After adding the dry ice acetone bath, open the branch valve, put in chlorotrifluoroethylene, keep the dry ice refluxed for 6 hours, and then pass the column with n-hexane to obtain a colorless liquid with a yield of 85%. 1 H NMR(300MHz, CDCl 3 , 293K, TMS) δ7.53-7.46 (m, 4H), 1.40 (s, 9H) ppm; 19 F NMR(282MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com