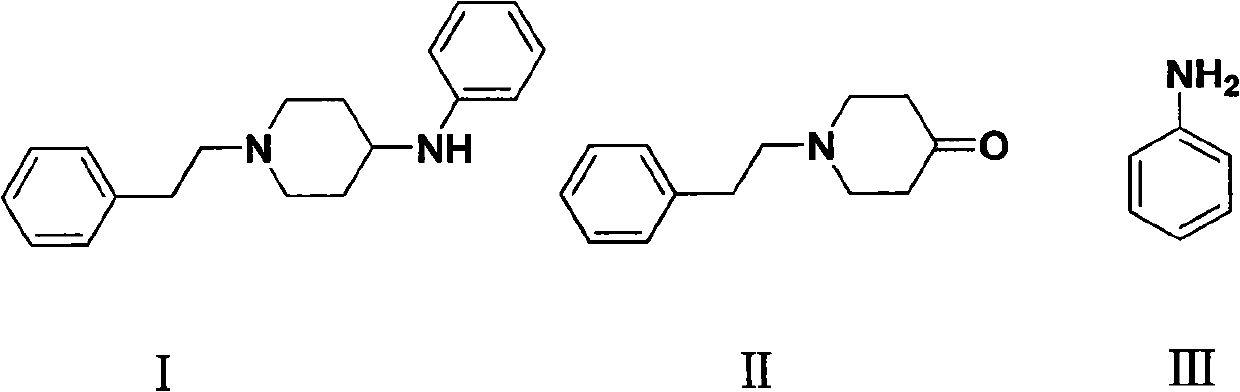
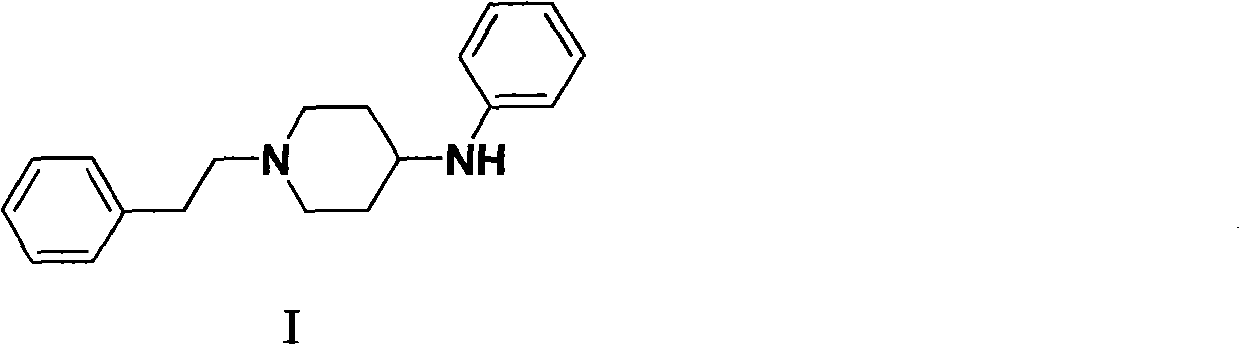
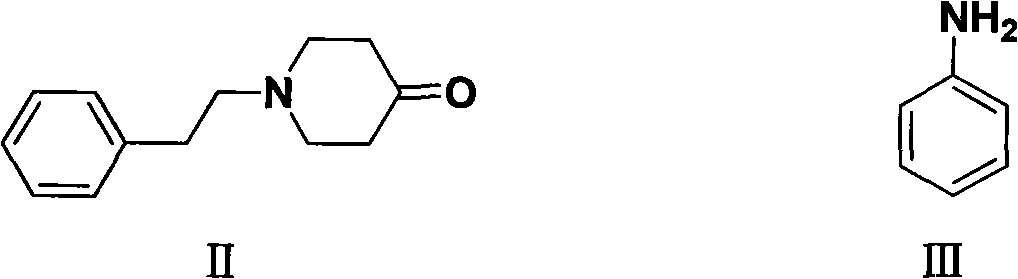
Preparation method of N-phenethyl-4-phenylaminopiperidine
A technology of anilinopiperidine and phenethyl, which is applied in the field of preparation of N-phenethyl-4-anilinylpiperidine, an intermediate of fentanyl, can solve the problem of harsh reaction conditions, high price and poor product quality. Stability and other issues, to achieve the effect of less by-products, low cost, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] In the preparation method of the present invention, thin layer chromatography (TLC) can be used to judge the end point of the preparation reaction (petroleum ether / ethyl acetate=10:1 (v / v)); and the obtained formula I The crude product of the compound shown can be purified by existing conventional purification methods such as recrystallization.
[0021] The method for preparing N-phenethyl-4-anilinopiperidine of the present invention has wide sources of raw materials, cheap and easy to obtain, few by-products, good product purity, high yield and low cost.
Embodiment 1
[0024] The preparation of N-phenethyl-4-piperidone (II):
[0025] Methyl acrylate (688.7g, 8.0mol) and anhydrous methanol (480ml) were mixed and stirred for 30min. A mixture of β-phenylethylamine (2) (387.8g, 3.2mol) and anhydrous methanol (320ml) was added dropwise under ice-cooling, and the internal temperature was controlled not to exceed 40°C. After the addition was complete, the temperature was raised to reflux and stirred for 8 hours. After cooling to room temperature, methanol and excess methyl acrylate were recovered under reduced pressure to obtain N,N-bis(β-methoxycarbonylethyl)phenethylamine (III) (926.0 g, yield 98.5%) as light yellow oily liquid.
[0026] 1 H NMR (500MHz, CDCl3) δ: 7.27(dd, J=9.29, 5.54Hz, 2H), 7.18(t, J=7.44, 7.44Hz, 3H), 3.66(s, 6H), 2.85(t, J= 7.14, 7.14 Hz, 4H), 2.70 (m, 4H), 2.45 (t, J = 7.13, 7.13 Hz, 4H). MS-EI (m / z, %): 293 (M+, 3.5), 189 (95), 146 (100), 91 (10).
[0027] Add toluene (300ml) and metal sodium wire (22.08g, 0.96mol) int...
Embodiment 2
[0030] Preparation of N-phenethyl-4-anilinopiperidine (I):
[0031] Add N-phenethyl-4-piperidone (54g, 0.266mol), aniline (27.54, 0.296mol), glacial acetic acid (3.0ml), dry 3A molecular sieves (75g), absolute ethanol to a 2L autoclave (1000ml), 3146 type Raney-Ni (20g), after purging the air in the kettle with nitrogen, feed hydrogen (pressure 0.4MPa), and react at 60°C for 2h. Cool down to room temperature, remove the solid by suction filtration to obtain a light yellow solution, distill off ethanol under reduced pressure, add petroleum ether (20ml), cool and crystallize, and obtain 65.6g of white crystal I by suction filtration, yield 88.1%, mp: 99-101 ℃; content 99.5% (HPLC);
[0032] 1 HNMR (400MHz) δ: 7.27(m, 2H), 7.19(dd, J=7.44, 3.43Hz, 3H), 7.15(m, 2H), 6.67(t, J=7.30, 7.30Hz, 1H), 6.58( d, J=7.76Hz, 2H), 3.65(m, 1H), 3.30(s, 1H), 2.94(d, J=11.70Hz, 2H), 2.80(dd, J=10.17, 6.31Hz, 2H), 2.60(m, 2H), 2.18(t, J=10.50, 10.50Hz, H), 2.07(d, J=12.23Hz, 2H), 1.49(dt, J=13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com