A kind of novel roxithromycin capsule and preparation method thereof
A technology of roxithromycin and capsules, which is applied in the field of new roxithromycin capsules and its preparation, can solve the problems of poor oral absorption and low bioavailability, and achieve simple operation, good reproducibility, and good curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of novel roxithromycin slow-release capsule comprises the component of following percentage by weight:
[0033] Roxithromycin 64%,
[0034] Hydroxypropyl Methyl Cellulose 4%,
[0035] Ethyl Cellulose 18%,
[0036] Dibutyl Sebacate 7%,
[0037] Polyethylene glycol (PEG1540) 6.2%,
[0038] Magnesium Stearate 0.8%.
[0039] The production method is as follows:
[0040] (1) Take roxithromycin and hydroxypropyl methylcellulose, add water of 10% (w / w) of its gross weight and mix evenly to make a soft material, granulate with a 20 mesh sieve, and dry at 60°C;
[0041] (2) After adding magnesium stearate and mixing, press to form a 2mm diameter drug chip;
[0042] (3) Weigh ethyl cellulose, dibutyl sebacate and polyethylene glycol (PEG1540), add its total weight 5% (w / w) to dissolve in water as coating solution;
[0043] (4) Carry out fluidized bed coating to the roxithromycin drug chip obtained in step (2) using the coating solution obtained in step (3), the air ...
Embodiment 2
[0046] A kind of novel roxithromycin slow-release capsule comprises the component of following percentage by weight:
[0047] Roxithromycin 68%,
[0048] Sodium Carboxymethyl Cellulose 5.5%,
[0049] Acrylic resin (Eudragit RS 30D-55) 15%,
[0050] Dibutyl Phthalate 6%,
[0051] Lactose 5%,
[0052] Talc 0.5%.
[0053] The production method is as follows:
[0054] (1) Take roxithromycin and sodium carboxymethyl cellulose, add water of 10% (w / w) of its gross weight and mix evenly to make soft material, granulate with 20 mesh sieve, and dry at 60°C;
[0055] (2) After adding talcum powder and mixing evenly, press it into a medicine chip with a diameter of 2mm;
[0056] (3) Acrylic resin (Eudragit RS 30D-55), dibutyl phthalate and lactose were weighed, and dissolved in water with a total weight of 5% (w / w) as a coating solution;
[0057] (4) Carry out fluidized bed coating to the roxithromycin drug chip obtained in step (2) using the coating solution obtained in step (3), th...
Embodiment 3
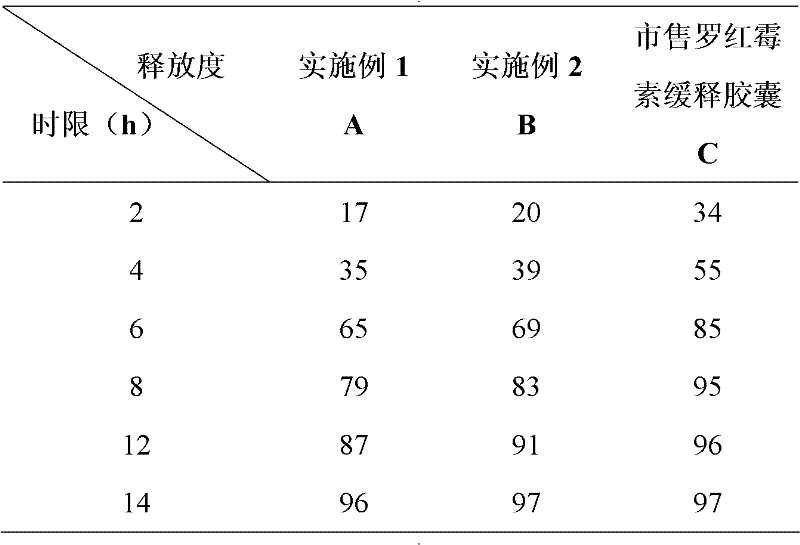
[0059] Example 3: In vitro release test.
[0060] Assay method: get the roxithromycin sustained-release capsules prepared in Examples 1 and 2 and the commercially available roxithromycin sustained-release capsules, according to the release degree (Appendix XD of Part Two of the Chinese Pharmacopoeia Edition in 2010). Adopt the second method device of dissolution assay (Chinese Pharmacopoeia 2010 edition two appendix XC) with hydrochloric acid solution (1→1000) 900ml as solvent, rotating speed is 50 revolutions per minute, operate according to law. At 2, 4, and 8 hours, 5ml of the solution was taken and filtered, and 5ml of solvent was added to the operating container immediately. Take appropriate amount of filtrate respectively, add hydrochloric acid solution to make a solution containing roxithromycin 60ug per 1ml as reference substance solution. Accurately measure each 4ml of the above solution and put it in a 10ml graduated test tube bottle, add 4ml of sulfuric acid soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com