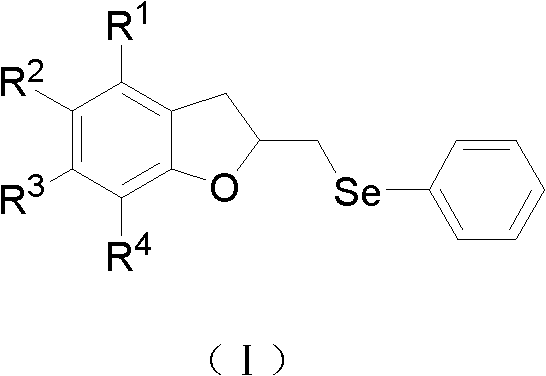
A kind of 2,3-dihydrobenzofuran derivative and its preparation and application
A derivative, dihydrobenzene technology, applied in 2 fields, can solve problems such as many side effects, psychotic episodes, liver toxicity, etc., and achieve the effects of easy industrialization, simple synthesis process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of 2-phenylselenylmethyl-4-hydroxy-5,7-dibenzoyl-2,3-dihydrobenzofuran (II)
[0051] The reaction formula is as follows:
[0052]
[0053] Under a nitrogen atmosphere, 7.16g (20mmol) of compound (II-2) was dissolved in 100mL of chloroform, and 4.96g (21mmol) of phenylselenium bromide (II-1), 2.80mL (20mmol) of triethylamine were added, Refluxing reaction for 5 hours, TLC detection compound (II-2) spot disappears, the reaction is complete, add 100mL saturated saline to separate layers, wash the organic phase with water 80mL×3, add anhydrous magnesium sulfate to dry, concentrate, and use petroleum ether and dichloro Methane (100:1) was recrystallized to obtain 9.234 g of white solid 2-phenylselenylmethyl-4-hydroxyl-5,7-dibenzoyl-2,3-dihydrobenzofuran (II), the yield 90%.
[0054] 1 H-NMR (CDCl 3 ): δ12.70(s, 1H), 7.78(s, 1H), 7.66-7.64(m, 2H), 7.54-7.534(m, 2H), 7.44-7.414(m, 4H), 7.36-7.30(m , 4H), 7.15-7.14(m, 3H), 5.06-5.03(m, 1H), 3.34-3....
Embodiment 2
[0056] Example 2: Preparation of 2-phenylselenylmethyl-4-propargyloxy-5,7-dibenzoyl-2,3-dihydrobenzofuran (IV)
[0057] The reaction formula is as follows:
[0058]
[0059] Under a nitrogen atmosphere, 5.12 g (10 mmol) of 2-phenylselenylmethyl-4-hydroxyl-5,7-dibenzoyl-2,3-dihydrobenzofuran (II) prepared in Example 1 ) was dissolved in 60mL acetone, added 1.4mL (15mmol) propargyl bromide (III), 2.1g (15mmol) potassium carbonate, reflux reaction for 5.0 hours, TLC tracking reaction, 2-phenylselenylmethyl-4-hydroxyl- The point of 5,7-dibenzoyl-2,3-dihydrobenzofuran (II) basically disappeared, the reaction was complete, the acetone was distilled off, extracted with 100mL dichloromethane, the organic phase was washed 3 times with saturated saline, and then Dry over anhydrous magnesium sulfate, concentrate, recrystallize the concentrate with a mixture of petroleum ether and dichloromethane (volume ratio: 100:1), filter, and dry to obtain 5.4 g of a white solid, namely 2-phenyls...
Embodiment 3
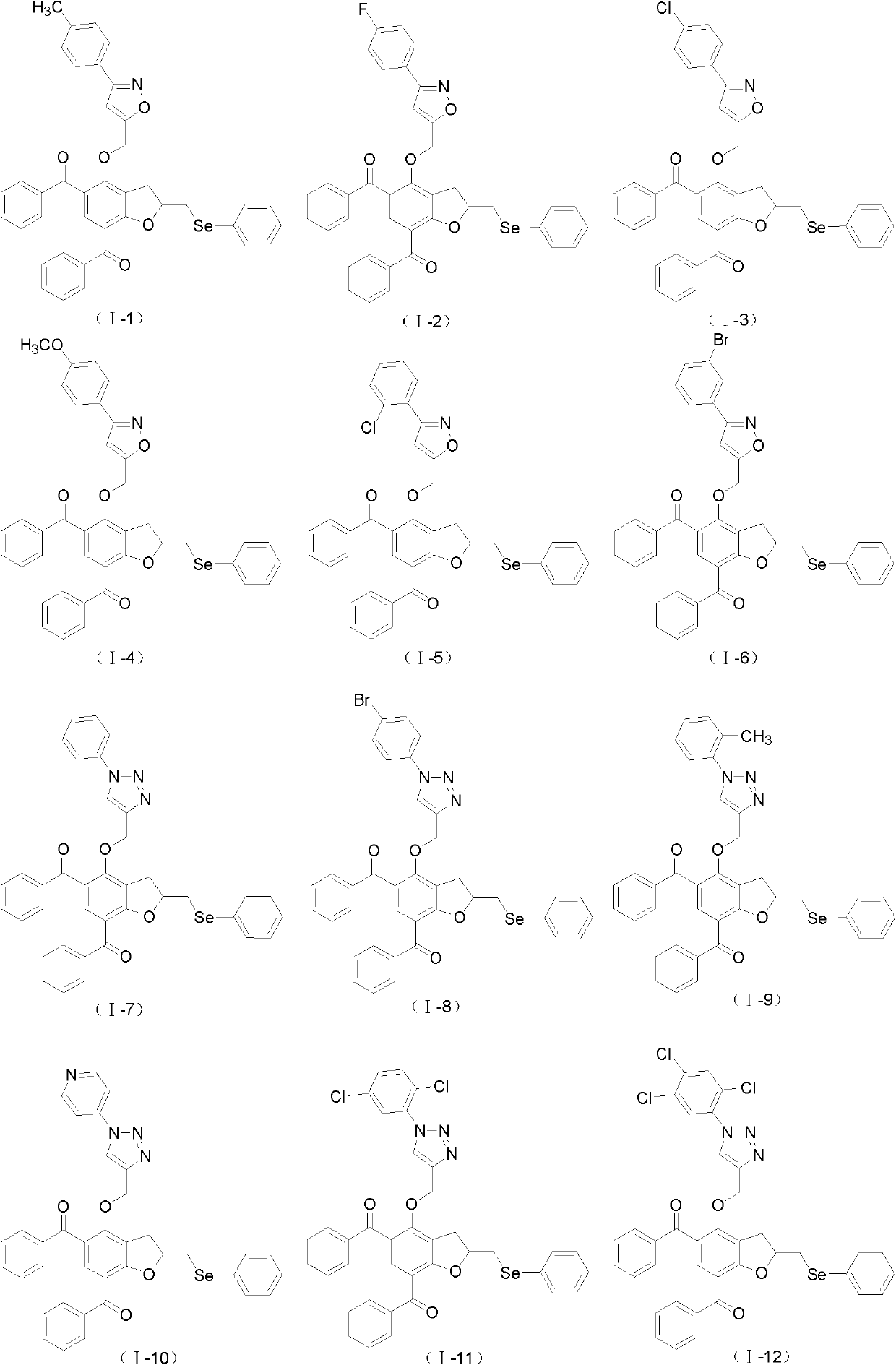
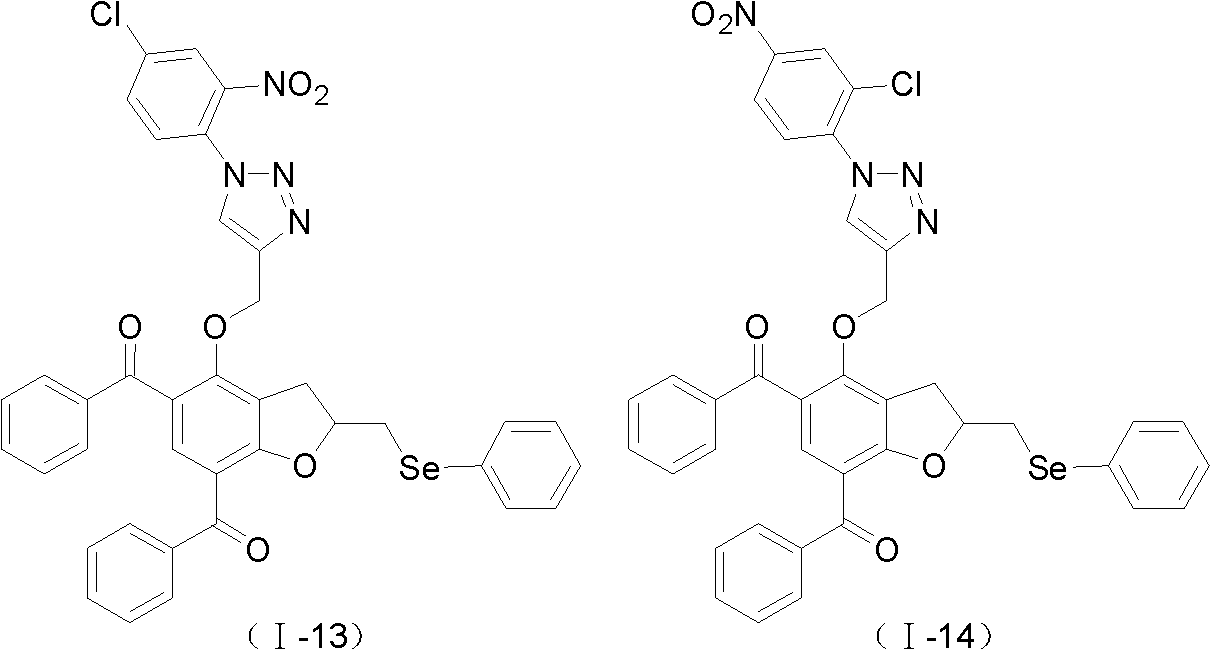
[0062] Example 3: 2-phenylselenylmethyl-4-(3-p-tolyl-isoxazol-5-ylmethoxy)-5,7-dibenzoyl-2,3-dihydrobenzene Preparation of furan (I-1)
[0063] The reaction formula is as follows:
[0064]
[0065] First, dissolve 0.540g (4.0mmol) 4-methylbenzaldehyde oxime (V-1) and 0.534g (4.0mmol) NCS in 30mL dichloromethane, and react at 30°C for 5 hours; under a nitrogen atmosphere, add 1.655 g (3.0mmol) 2-phenylselenylmethyl-4-propargyloxy-5,7-dibenzoyl-2,3-dihydrobenzofuran (IV) prepared by the method in Example 2, 2.0 hours 0.70mL triethylamine (5mmol, dissolved with 5mL dichloromethane) was added dropwise thereto, and then incubated at 30°C for 8 hours, the compound point shown in TLC (IV) disappeared and the reaction was complete, extracted with 40mL dichloromethane, organic Add 60mL of saturated saline solution to wash twice, then dry with anhydrous magnesium sulfate, concentrate, and the concentrate is passed through a chromatographic column (eluent is V 石油醚 :V 乙酸乙酯 =1:5), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com