Preparation method of titanium dioxide transparent aqueous sol doped with metal ions
A metal ion, titanium dioxide technology, applied in the direction of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc., can solve the problem of limiting the utilization of sunlight, and achieve the improvement of photocatalytic efficiency, avoid pollution, and good photocatalytic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
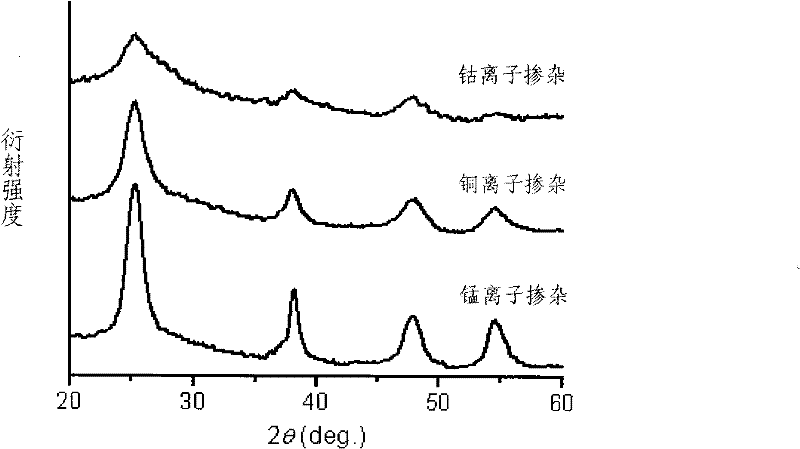
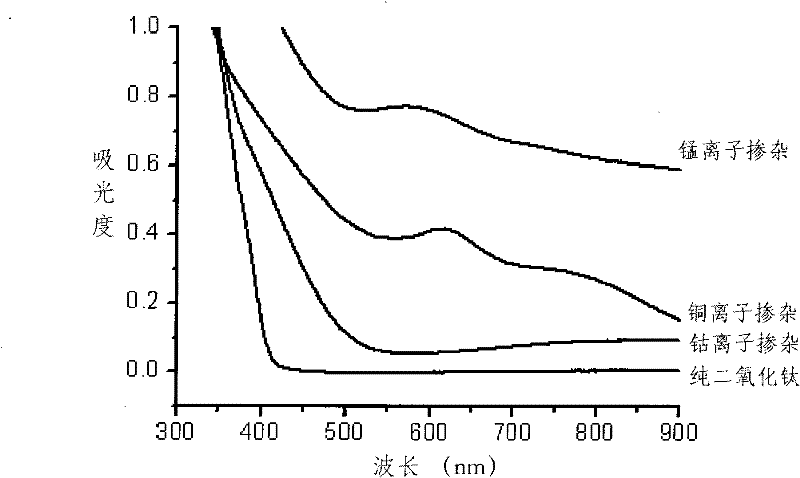
[0023] 0.01mol manganese sulfate (MnSO 4 ) and 0.1mol titanium tetrachloride were dissolved in 500mL water to form a manganese-titanium mixed solution, and ammonia solution was added dropwise to the mixed solution until the pH was 10 to obtain a manganese-titanium composite titanic acid precipitate, which was washed with water and filtered to remove sulfuric acid root and chloride ions; the washed and filtered precipitate is dispersed in 50 mL of 30% hydrogen peroxide solution (the molar ratio of hydrogen peroxide to titanium ions in the composite titanic acid precipitate is 5), heated to reflux at 90°C 4 hours, obtain the titania sol of manganese ion doping (in kind see figure 1 ), the pH value is between 7.5 and 8.0.
Embodiment 2
[0025] 0.0001mol copper acetate (Cu(CH 3 COO) 2 ) and 0.1mol titanium tetrachloride were dissolved in 500mL water to form a copper-titanium mixed solution, and ammonia solution was added dropwise to the mixed solution until the pH was 10 to obtain a copper-titanium composite titanic acid precipitate, which was washed with water and filtered to remove the acetic acid therein root and chloride ions; the washed and filtered precipitate is dispersed in 60 mL of 5% hydrogen peroxide solution (the molar ratio of hydrogen peroxide to titanium ions in the composite titanic acid precipitate is 1), heated to reflux at 90°C 1 hour, obtain the titania sol of copper ion doping (in kind see figure 1 ), the pH value is between 6.0 and 6.5.
Embodiment 3
[0027] 0.005mol cobalt nitrate (Co(NO 3 ) 2 ) and 0.1mol titanyl sulfate were dissolved in 500 mL water to form a cobalt-titanium mixed solution, and an aqueous sodium hydroxide solution was added dropwise to the mixed solution until the pH was 8 to obtain a cobalt-titanium composite titanic acid precipitate, which was washed with water and filtered to remove the Sulphate and nitrate ions; the washed and filtered precipitate is dispersed in 200mL of mass concentration of 15% hydrogen peroxide solution (the molar ratio of hydrogen peroxide to titanium ions in the composite titanic acid precipitate is 10), 100°C Heated and refluxed for 2 hours to obtain the titanium dioxide sol doped with cobalt ions (see figure 1 ), the pH value is between 6.5 and 6.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com