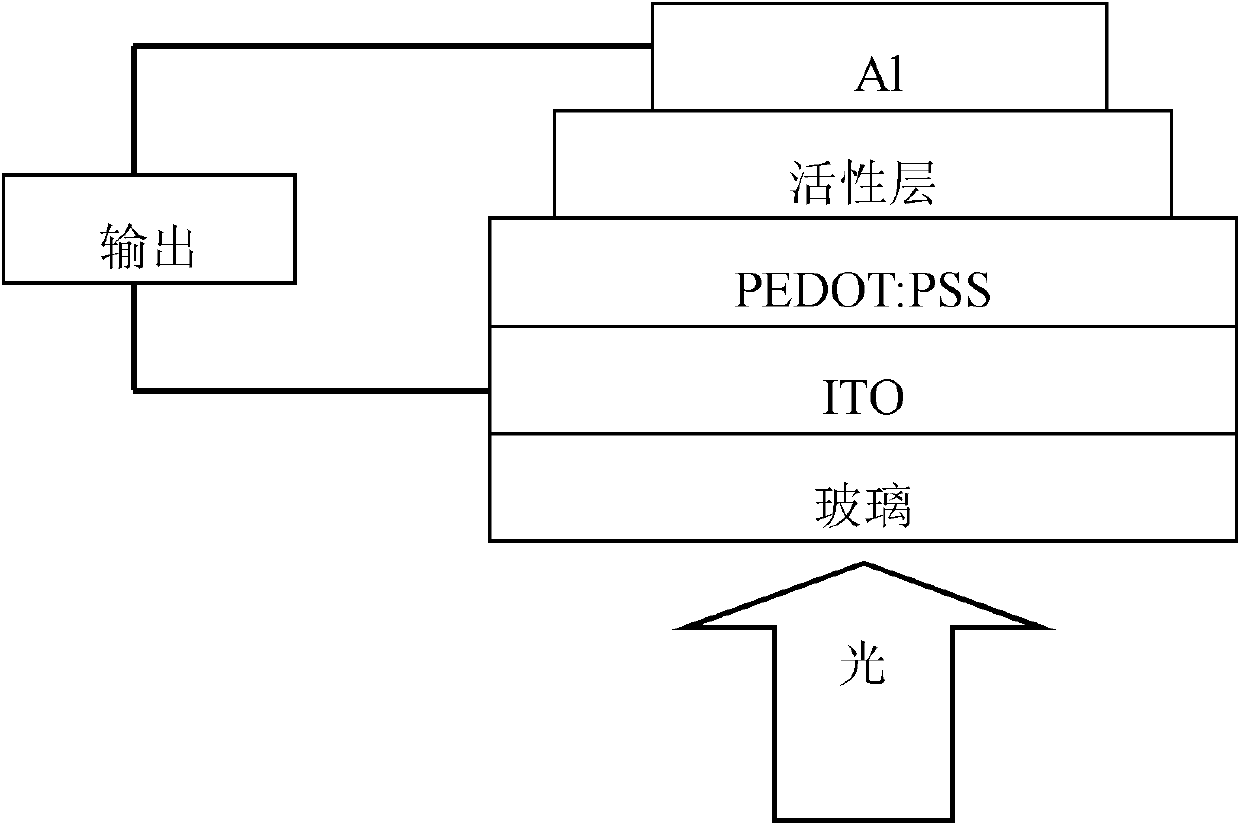
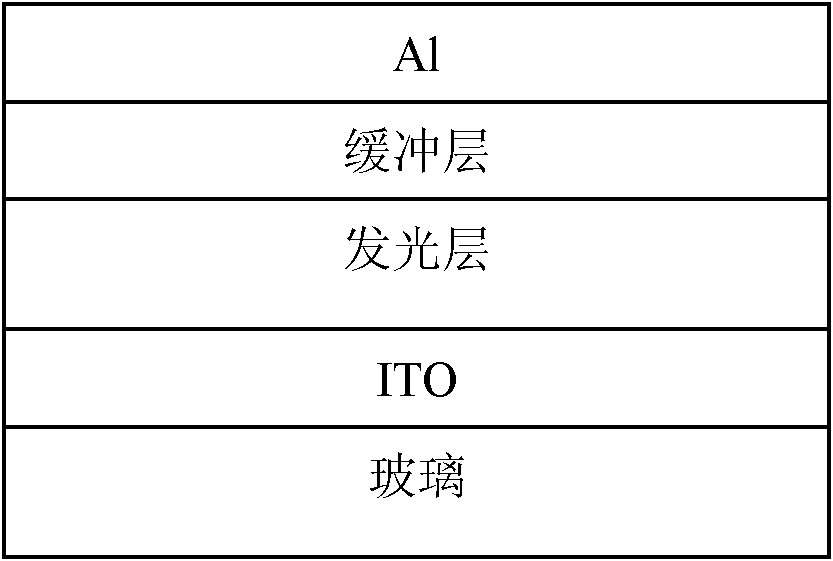
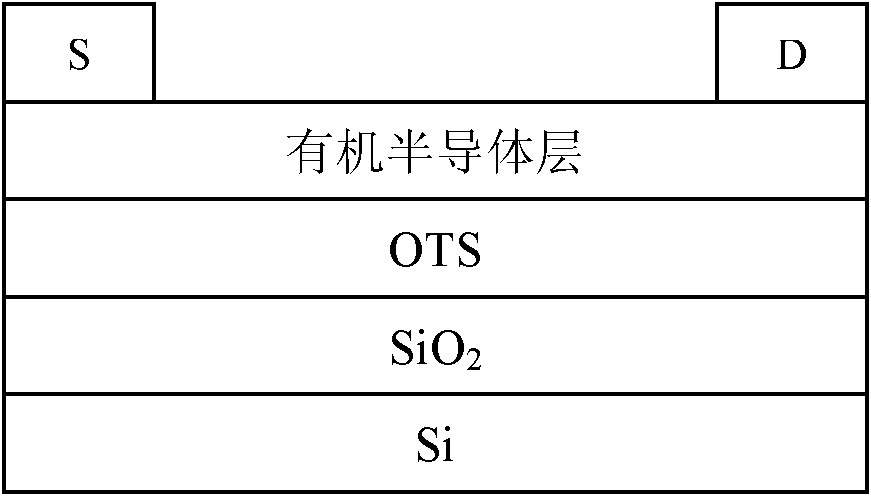
Carbazole porphyrin-paranaphthalene copolymer and preparation method and application thereof
A carbazole porphyrin and copolymer technology, which is applied in the field of polymer materials, can solve the problems of solar radiation spectrum mismatch, low carrier mobility, low photoelectric conversion efficiency, etc., and achieves high carrier mobility and spectrum. Wide absorption, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Preparation of Carbazole-Containing Porphyrin-Anthracene Copolymer
[0034] Carbazole-containing porphyrin-anthracene copolymer of the present invention can be prepared by the method shown in the following formula:
[0035]
[0036] In the formula, R 1 and R 2 same or different as H or C 1 ~C 32 Alkyl, phenyl, containing one or more of the same or different C 1 ~C 32 In the substituted phenyl group of an alkyl group or an alkoxy group, n is an integer of 1-100.
[0037] Under the protective conditions of inert gases such as nitrogen and argon, add 5,15-dibromo-10,20-alkylcarbazole porphyrin, bisborate anthracene, catalyst and alkali solution into the organic solvent, The Suzuki reaction was carried out at ℃ for 24-72 hours to obtain a carbazole-containing porphyrin-anthracene copolymer. In this reaction, preferably, the catalyst is a mixture of organopalladium and organophosphine ligands, Pd 2 (dba) 3 / P(o-Tol) 3 , Pd(PPh 3 ) 4 or Pd(PPh 3 ) 2 Cl 2 Etc. ...
Embodiment 1
[0040] Example 1 10,20-two (9-octylcarbazole) porphyrin-anthracene copolymer, its chemical formula is as follows:
[0041]
[0042] In the formula, n=37.
[0043] 1. Preparation of 9,10-p-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) anthracene
[0044]
[0045] in N 2 Under the protection of the three-necked flask, 10 g of p-9,10-dibromoanthracene and 250 mL of tetrahydrofuran (THF) solvent were added, and then 2.5 M n-butyllithium (n-BuLi )25.2mL, continue stirring for 2 hours, then inject 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborol with a syringe at -78°C Alkane 13mL, stirred at room temperature for 6-16 hours. The reaction was terminated by adding saturated aqueous sodium chloride solution, extracted with chloroform, dried over anhydrous sodium sulfate and filtered with suction, the filtrate was collected and the solvent was removed by rotary evaporation. Finally, the crude product was separated by silica gel column chromatography using petroleum ether / ethyl...
Embodiment 2
[0058] Example 2 10-(9'-methylcarbazole)-20-(9'-docosylcarbazole) porphyrin-anthracene copolymer, its chemical formula is as follows:
[0059]
[0060] In the formula, n=25.
[0061] 1. Preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)anthracene
[0062] The preparation steps are detailed in Example 1.
[0063] Two, the preparation of 5-(9'-methylcarbazole)-15-(9'-docosylcarbazole) porphyrin
[0064]
[0065] Set up an anhydrous and oxygen-free device, weigh intermediate 2-aldehyde-9-methylcarbazole (0.21g, 1mmol), 2-aldehyde-9-docosylcarbazole (0.65g, 1mmol) and di Pyrromethane (0.30g, 2mmol) was dissolved in 250mL of dichloromethane, nitrogen gas was introduced for 30min, 2mL of trifluoroacetic acid was added into the syringe, stirred at 100°C for 1h, then DDQ (1.82g, 8mmol) was added, and the Stir for 30 min, then add 2 mL of pyridine to quench the reaction, concentrate the solvent, filter, collect the filtrate and remove the solvent by rotary evapor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com