Dithiophene-containing thiophene-thiophene-quinoxaline conjugated polymer and its preparation method and application
A technology of conjugated polymer and thiophene thiorole, which is applied in the field of organic compound synthesis, can solve the problems of underutilized red light region, low collection efficiency of carrier electrodes, low carrier mobility, etc., and achieve excellent electrical properties. Effects of chemical reduction properties, good thermal and environmental stability, and high electron transport properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] This embodiment also provides a method for preparing the above-mentioned conjugated polymer containing dithiophene thiazole-thiophene-quinoxaline, including the following steps:
[0043] 1) Provide compounds A, B, C, and D represented by the following structural formula respectively,
[0044]
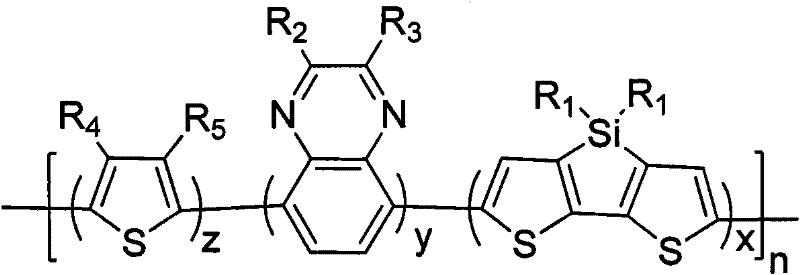
[0045] Among them, R 1 from C 1 ~C 20 the alkyl group; R 2 , R 3 selected from -H, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy groups, alkyl or alkoxy benzene ring groups, alkyl fluorene groups or alkyl carbazole groups; R 4 , R 5 selected from -H, C 1 ~C 20 Alkyl or C 1 ~C 20 alkoxy;
[0046] 2) In an oxygen-free environment and in the presence of a catalyst and an organic solvent, select compounds A, B, C, and D to carry out a Stille coupling reaction to obtain a dithiophene-containing thiazole-thiophene- Quinoxaline conjugated polymers. The Stille coupled chemical reaction formula of step 2) can be expressed as follows:
[0047]
[0048] In the general structural ...
Embodiment 1
[0057] Dithiophene [3,2-b: 2', 3'-d] silole-thiophene-2, the preparation of 3 two (phenyl substituted) quinoxaline conjugated polymers, its structural formula is as follows I 1 as shown,
[0058]
[0059] Its preparation steps are as follows:
[0060] 1) The preparation of compound 2,5-dibromo-4,5 dioctylthiophene, its chemical reaction formula is as follows:
[0061]
[0062] The specific process of preparation is: NBS (2.9g, 16.3mmol) was added to the DMF (50mL) solution containing 3,4-dioctylthiophene (2.1g, 6.8mmol), stirred at room temperature for 12 hours, and the reaction solution was poured into into saturated brine, followed by chloroform extraction, saturated brine washing, rotary evaporation to remove the solvent to obtain the crude product, the crude product was obtained by column chromatography (petroleum ether as eluent) to obtain an oily product, the yield was 76%, and the MS (EI) m / z of the product: 466 (M + ).
[0063] 2) The preparation of compound ...
Embodiment 2
[0073] Dithiophene [3,2-b: 2', 3'-d] silole-thiophene-2, the preparation of 3 two (phenyl substituted) quinoxaline conjugated polymers, its structural formula is as follows I 2 as shown,
[0074]
[0075] Its preparation steps are as follows:
[0076] 1) According to the same preparation method and similar reaction conditions of step 1) in Example 1, the compound 2,5-dibromo-3,4-dioctyloxythiophene with the following structural formula was prepared,
[0077]
[0078] 2) According to the same preparation method and similar reaction conditions in step 2) in Example 1, 5,8-dibromo-2-(4-n-butylphenyl) 3-(4-eicosane) with the following structural formula was prepared Oxyphenyl)quinoxaline,
[0079]
[0080] 3) According to the same preparation method and similar reaction conditions as step 3) in Example 1, 4,4'-di-n-butyl-2,6-bistrimethyltin-dithiophene [3,2 -b:2',3'-d] silole,
[0081]
[0082] 4) Cyclopentadiene (2,1-b:3,4-b') dithiophene-thiophene-2,3 bis(phenyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com