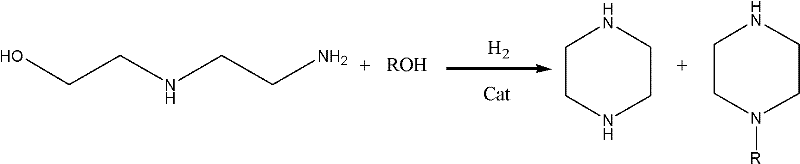Process for coproducing piperazine and N-alkyl piperazine
A technology for alkylpiperazine and piperazine, which is applied in the field of co-production technology of piperazine and N-alkylpiperazine, can solve the problems of low yield, unknown synthesis process, low production efficiency and the like, and achieves the yield of synthetic products High and simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The reactor is a fixed-bed tubular reactor with an inner diameter of 25 mm, a tube length of 470 mm, and a built-in catalyst of 60 g. The catalyst contains 5% nickel, 4% copper, 3% chromium, and 1% zinc. The carrier is zirconia (ZrO 2 ). Mix N-β-hydroxyethylethylenediamine with a mass ratio of 40% and ethanol, preheat for 250h -1 The speed enters the catalyst bed layer with a temperature of 180°C, the hydrogen pressure in the tube is 8Mpa, and the product is analyzed by gas chromatography (using SC-54 capillary column, column temperature 180°C, vaporization chamber temperature 220°C, detector temperature 220°C). analyze.
[0020] Gas chromatographic analysis results: the conversion rate of N-β-hydroxyethylethylenediamine is 99.2%, the selectivity of piperazine is 85.3%, the selectivity of N-ethylpiperazine is 8.1%, N, N'-diethyl The selectivity to piperazine was 0.8%.
Embodiment 2
[0022] In the fixed-bed reactor packing 55g catalyst of embodiment 1, the composition of catalyst is to contain cobalt 10%, copper 5%, chromium 5%, iron 2%, and carrier is aluminum oxide (Al 2 o 3 ). Mix N-β-hydroxyethylethylenediamine and isopropanol with a mass ratio of 20%, preheat for 1000h -1 The speed enters the catalyst bed layer that temperature is 180 ℃, and hydrogen pressure is 10Mpa, utilizes gas chromatography (condition to be the same as embodiment 1) to analyze product.
[0023] Gas chromatography analysis results: the conversion rate of N-β-hydroxyethylethylenediamine is 98.2%, the selectivity of piperazine is 80.3%, the selectivity of N-isopropyl piperazine is 11.3%, N, N'-di The selectivity to isopropylpiperazine was 5.1%.
Embodiment 3
[0025] Fill 70g catalyst in the fixed-bed tubular reactor of embodiment 1, the composition of catalyst is to contain copper 5.5%, chromium oxide 3%, zinc oxide 5%, and carrier is titanium oxide (TiO 2 ). Mix N-β-hydroxyethylethylenediamine with a mass ratio of 50% and methanol, preheat for 200h -1 The speed enters the catalyst bed layer that temperature is 230 ℃, and hydrogen pressure is 4.8Mpa, utilizes gas chromatography (condition to be the same as embodiment 1) to analyze product.
[0026] Gas chromatographic analysis results: the conversion rate of N-β-hydroxyethylethylenediamine is 98.8%, the selectivity of piperazine is 90.3%, the selectivity of N-methylpiperazine is 6.7%, N, N'-dimethyl The selectivity to piperazine was 1.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com