Three fluorescence complexes formed on basis of Eu (III) and chlorosulfonation four-tooth Beta-diketone ligands and application thereof
A technology for chlorosulfonylation of tetradentate and ligands, which is applied in the field of fluorescent complexes, can solve the problems of limited probe types, expensive probe reagents, and restrictions on the development and application of time-resolved fluorescence biochemical analysis techniques, achieving cost Effects of low, high stability, and long fluorescence lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
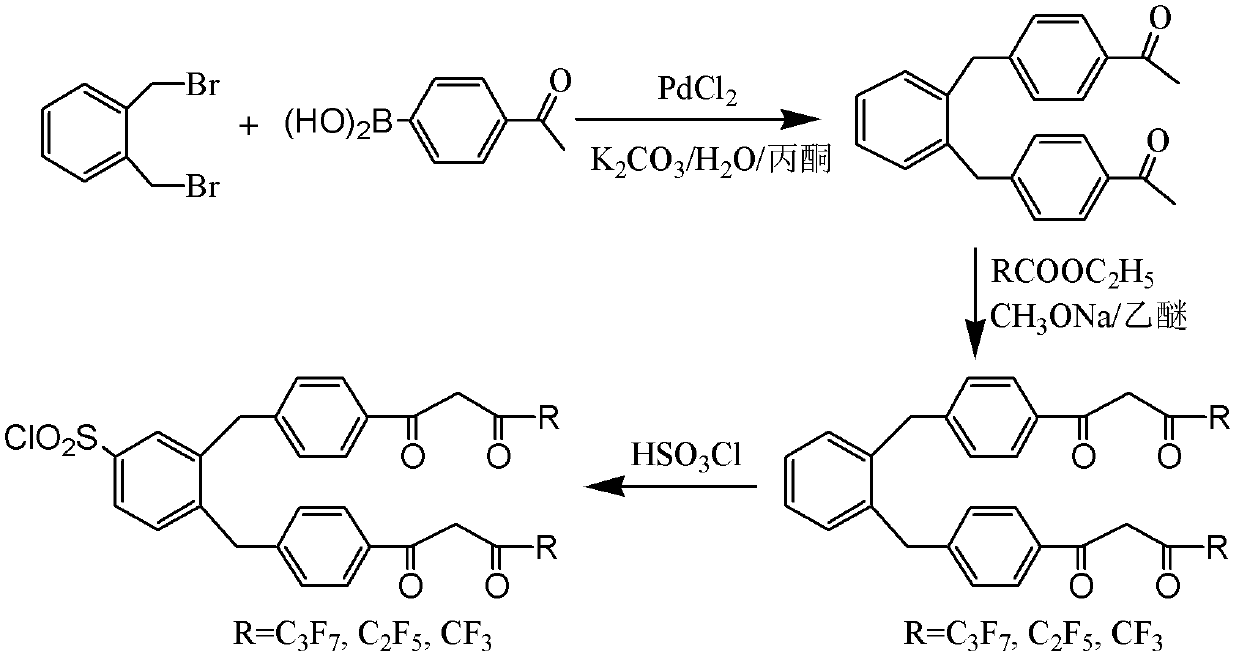
[0028] Example 1: Synthesis of three chlorosulfonylated tetradentate β-diketone ligands BHHBCB, BPPBCB and BTBBCB
[0029] The synthetic routes of three chlorosulfonylated tetradentate β-diketone ligands are as follows: figure 1 As shown, the synthetic processes of the three ligands are similar, only in the 1,2-bis(fluoroalkyl-β-diketonyl-p-benzyl)benzene synthesis step, the type of ethyl fluoroalkylcarboxylate used The specific experimental process is as follows.
[0030] (1) Synthesis of 1,2-bis(4'-acetylbenzyl)benzene.
[0031] Add 2.64g (10mmol) of 1,2-di(bromomethyl)benzene, 5.91g (36mmol) of 4-acetylphenylboronic acid, and 6.90g (50mmol) of potassium carbonate into a round-bottomed flask containing 60mL of acetone and 20mL of water, Stir under cooling in an ice-water bath until most of it dissolves, then add 70.90 mg (0.4 mmol) of palladium chloride, heat to 50° C. under argon protection, and stir for 12 hours. After the reaction, the solvent was evaporated under redu...
Embodiment 2
[0036] Example 2: Preparation of Europium(III) complexes of three chlorosulfonylated tetradentate β-diketone ligands to label bovine serum albumin
[0037] The chlorosulfonyl groups contained in the three ligands can react with amino-containing biomolecules to form sulfonamide covalent bonds and be labeled on biomolecules. In order to investigate the use of three chlorosulfonylated tetradentate β-diketone ligands for The labeling conditions of actual biomolecules and the fluorescent properties of the complexes formed by the three ligands and europium (III) after the biomolecules are labeled. In this example, bovine serum albumin (BSA for short) was used as a model biomolecule to prepare BHHBCB, The BSA solution labeled with the trivalent europium complex of BPPBCB and BTBBCB, the specific experimental method is as follows.
[0038] After dissolving 10.0 mg of BSA in 2.0 mL of 0.05 mol / L sodium carbonate buffer solution with a pH value of 9.3, slowly add 9.0×10 -3 A solution o...
Embodiment 3
[0040] Example 3: Measurement of Fluorescence Spectra and Fluorescent Properties of Three Europium (III) Complexes Labeled BSA
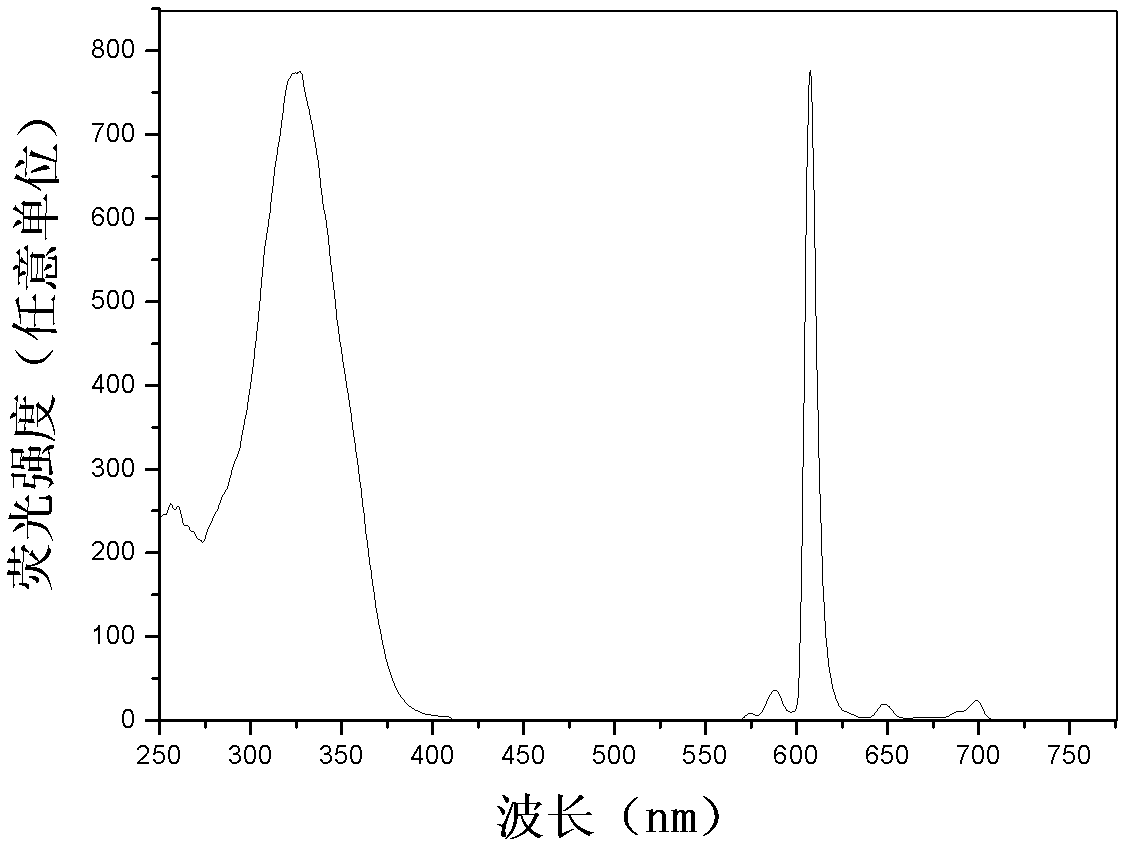
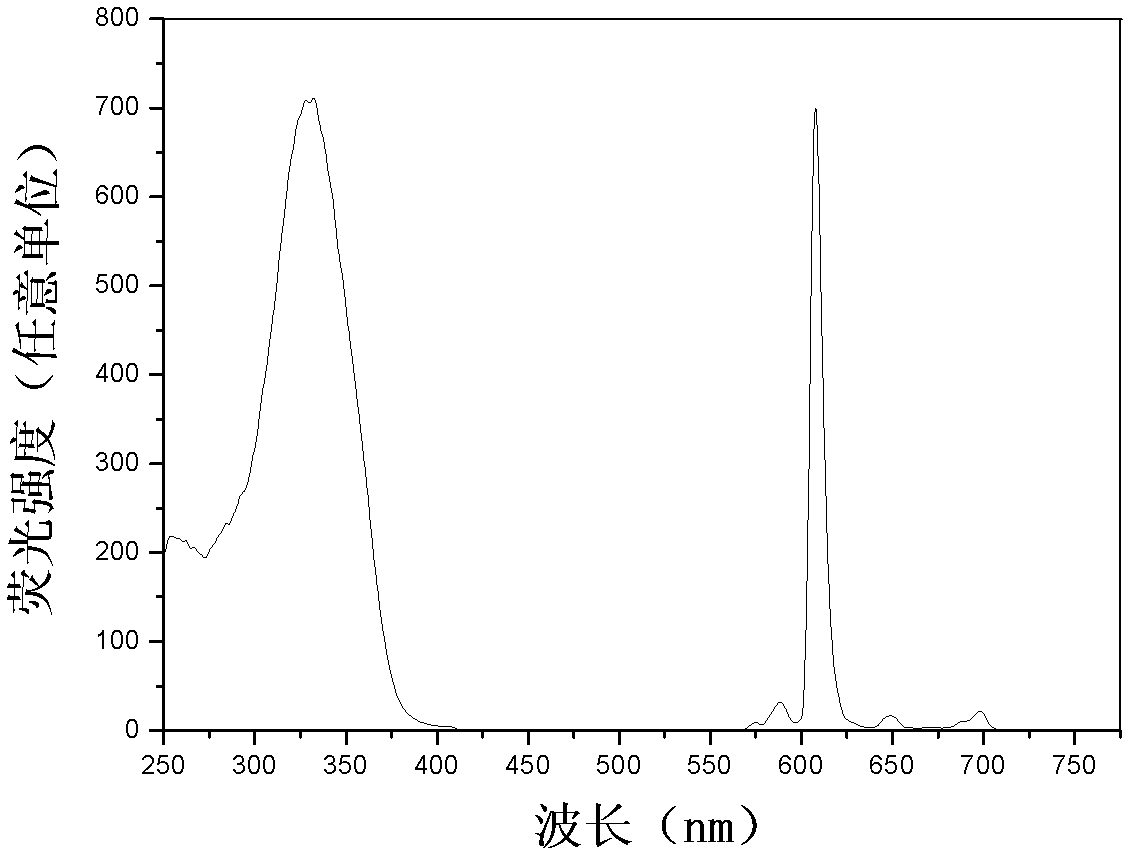
[0041] Dilute the three kinds of europium (III) complex-labeled BSA solutions to an appropriate concentration with 0.05mol / L boric acid buffer solution with a pH value of 9.1, and then measure the steady-state fluorescence excitation and emission spectra and fluorescence quantum yields (φ ) and fluorescence lifetime (τ), the results are shown in Table 1.
[0042] Table 1. Fluorescence properties of BSA solutions labeled with three europium(III) complexes
[0043]
[0044] figure 2 , image 3 and Figure 4 BHHBCB-Eu are given respectively 3+ 、BPPBCB-Eu 3+ and BTBBCB-Eu 3+ The time-resolved fluorescence excitation and emission spectra of the labeled BSA solution diluted with 0.05mol / L Tris-HCl buffer solution with a pH value of 7.8. The measurement conditions are: delay time, 0.2ms; counting window time, 0.4ms; cycle time, 20ms; Excitation s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com