High-temperature-resistant Pyrolobus polymerase and efficient expression plasmid and application thereof
A high-efficiency expression, polymerase technology, applied in applications, transferases, botanical equipment and methods, etc., can solve problems such as slow speed, and achieve the effects of strong high temperature resistance, good practicability, good economic benefits and social effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Depend on Pyrolobus fumarii The hyperthermophilicity infers that its polymerase has high thermotolerance, using the known Pyrococcus furiosus DNA polymerase protein sequence (NP_577941.1) as target in Pyrolobus fummarii Query in the genome of Pyrfu_0295 and called out the encoded protein of Pyrfu_0295 (YP_004780419.1), its protein sequence contains 803 amino acids, and the specific sequence is shown in sequence 1 in the sequence listing. Structural analysis of the Pyrfu_0295 protein, discovery and Pyrococcus furiosus DNA polymerases share 36% identical amino acid sequence and 55% similar amino acid. In addition to its carbon-terminal polymerase domain, it also includes a 3' to 5' exonuclease region at its amino-terminus that can help detect gene replication errors. It was preliminarily identified that Pyrolobus polymerase has similar functions to Pyrococcus polymerase.
[0024] The combination of polymerase and DNA is the rate-limiting step of the entire catalyt...
Embodiment 2
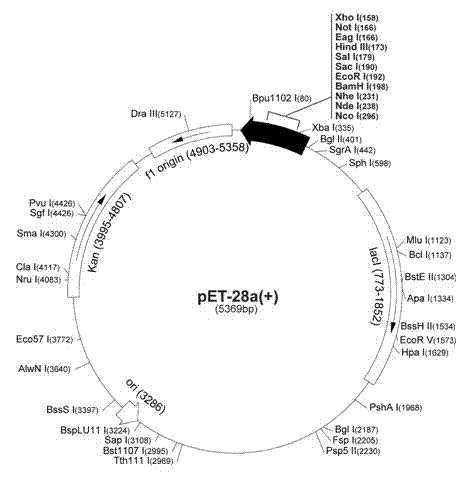
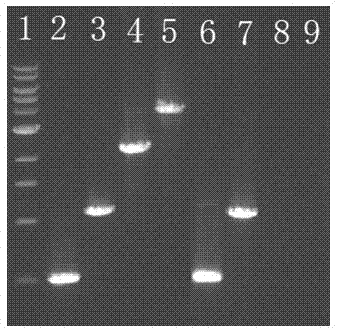
[0026] There are various technical obstacles to cultivating thermophiles, such as their inability to grow on solid gels, the co-growth of archaeal cells into a network structure, and the almost non-existent fragility of individual cells, which makes fermentation difficult and expensive in the laboratory. In addition, the expression of the original polymerase in Pyrolobus is low, which will further increase the cost. Recombinant protein protein expression and purification technology has been widely used in various enzyme technologies. Mass cultureable hosts include bacteria, fungi, plants or eukaryotic cells. Escherichia coli will be used to express the Pyrolobus polymerase in large quantities in the present invention. But because each organism has its most suitable coding table, when the original Pyrolobus polymerase gene was expressed in E. coli, the protein expression was so low that it was difficult to detect. In order to increase the expression level of Pyrolobus polymer...
Embodiment 3
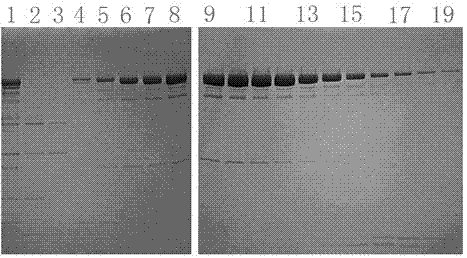
[0037] The correct expression plasmid identified by sequencing in Example 2 was transformed into T7 expression strain BL21(DE3), spread on an ampicillin selection plate, and cultured overnight at 37°C. Pick an independent colony into 10mL liquid medium containing 100μg / mL ampicillin, culture at 37°C until OD 600 After reaching 0.4~0.6, add 40 μL of 100 mM IPTG (Sigma I5502) stock solution (final concentration is 0.4 mM), induce for 4 hours at 37°C, grow for 5 hours at 30°C or grow overnight at 16°C. A 40 μL sample was taken for 10-20% Tris-glycine SDS-PAGE (Invitrogen Cat#EC61352BOX) electrophoresis analysis, and the expression was detected by Coomassie brilliant blue stained protein gel and Western Blot, and it was found that the expression level was the highest after induction at 37°C for 3 hours. For large-scale expression, take fresh 10mL culture medium and inoculate it into 10L liquid medium (containing antibiotics), culture at 37°C until OD 600 After reaching 0.4-0.6, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com