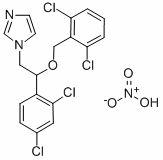
Method for preparing isoconazole nitrate
A technology of isoconazole nitrate and aluminum isopropoxide, applied in the field of medicine and chemical industry, can solve the problems of harsh conditions, danger, high price, etc., and achieve the effects of mild reaction conditions, improved operability, and reduced product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
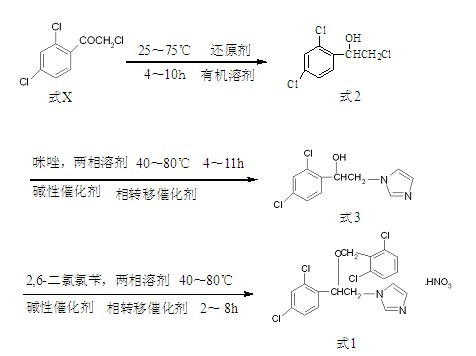
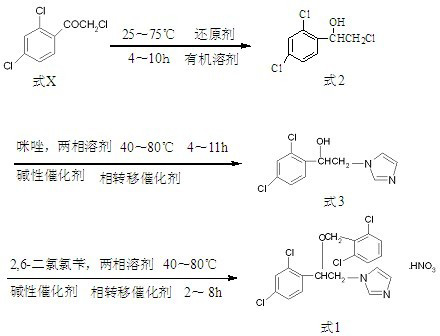
[0031] In a 2000mL three-necked reaction flask with mechanical stirring and reflux condenser, add 200g (0.89mol) of trichloroacetophenone, 1200mL of isopropanol and 80g (0.39mol) of aluminum isopropoxide, stir, and heat to 75°C for reaction 7 Hour. Stop the reaction, and concentrate the reaction liquid in the reaction flask to oil under reduced pressure to obtain 1-(2,4-dichlorophenyl)-2-chloro-ethanol.
[0032] Put 1-(2,4-dichlorophenyl)-2-chloro-ethanol, water 200mL, sodium hydroxide 315g, toluene 1000mL, imidazole 106g (1.56mol) and triethylbenzyl ammonium chloride 32g into In the reaction bottle, after 7 hours of heat preservation at 60°C, stop the reaction, separate the reacted mixture, wash the organic phase with water, then cool to 0°C, precipitate crystals, then filter, and recrystallize the filter cake with 95% ethanol. 1-[2-(2,4-Dichlorophenyl)-2-hydroxyethyl]imidazole is obtained.
[0033] Mix 1-[2-(2,4-dichlorophenyl)-2-hydroxyethyl]imidazole, water 360 mL, sodiu...
Embodiment 2
[0036] In a 2000mL reactor, add 200g (0.89mol) of trichloroacetophenone, 1000mL of methanol and 20g (10%wt) of Raney nickel, pressurize to 1MPa, react at 25°C for 4 hours, and stop the reaction. The reaction solution was concentrated to oil under reduced pressure to obtain 1-(2,4-dichlorophenyl)-2-chloro-ethanol.
[0037] Put 1-(2,4-dichlorophenyl)-2-chloro-ethanol, water 200mL, sodium hydroxide 295g, toluene 900mL, imidazole 98g (1.44mol) and triethylbenzyl ammonium chloride 30g into In the reaction flask, keep warm at 60°C for 7 hours, stop the reaction, separate the reacted mixture, wash the organic phase with water, then cool to -5°C, crystallize, then filter, and recrystallize the filter cake with 95% ethanol , to give 1-[2-(2,4-dichlorophenyl)-2-hydroxyethyl]imidazole.
[0038] Mix 1-[2-(2,4-dichlorophenyl)-2-hydroxyethyl]imidazole, water 330 mL, sodium hydroxide 257 g, toluene 1000 mL, triethylbenzyl ammonium chloride 14.2 g (0.06 mol) and 114.5 g (0.59 mol) of 2,6-di...
Embodiment example 3
[0041] In a 2000mL three-necked reaction flask with mechanical stirring and reflux condenser, add 200g (0.89mol) of trichloroacetophenone, 1200mL of isopropanol and 80g (0.39mol) of aluminum isopropoxide, stir, and heat to 75°C for reaction 7 Hour. Stop the reaction, and concentrate the reaction liquid in the reaction flask to oil under reduced pressure to obtain 1-(2,4-dichlorophenyl)-2-chloro-ethanol.
[0042] Put 1-(2,4-dichlorophenyl)-2-chloro-ethanol, water 500mL, sodium carbonate 835g, toluene 1000mL, imidazole 106g (1.56mol) and tetrabutylammonium bromide 45.3g into the reaction bottle in sequence , after 7 hours of heat preservation at 60°C, stop the reaction, separate the reacted mixture, wash the organic phase with water, then cool to -10°C, crystallize, then filter, and recrystallize the filter cake with 95% ethanol to obtain 1-[2-(2,4-Dichlorophenyl)-2-hydroxyethyl]imidazole.
[0043] Mix 1-[2-(2,4-dichlorophenyl)-2-hydroxyethyl]imidazole, water 450mL and sodium ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com