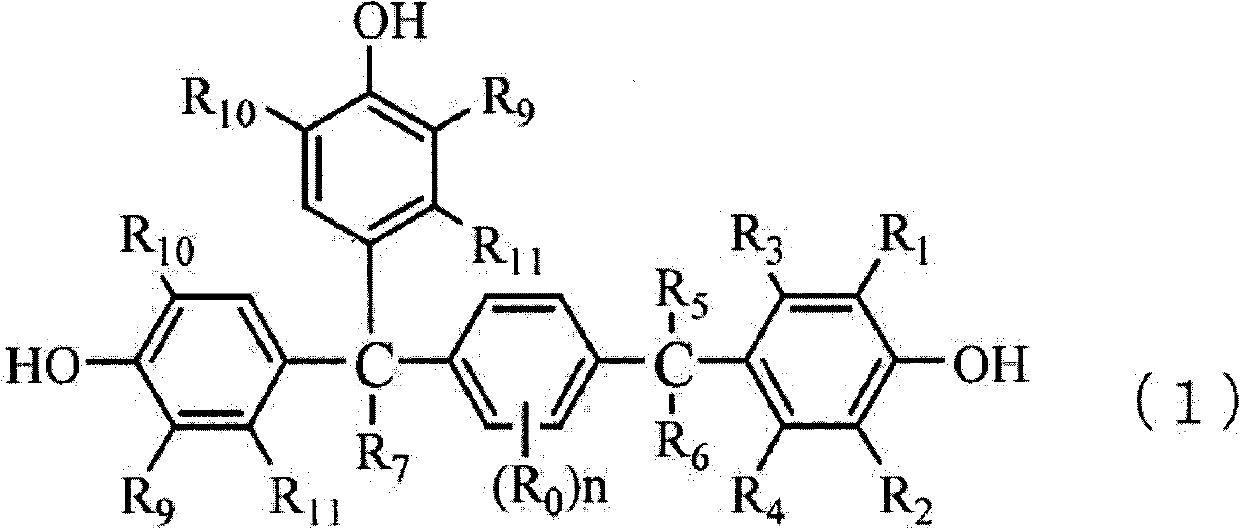
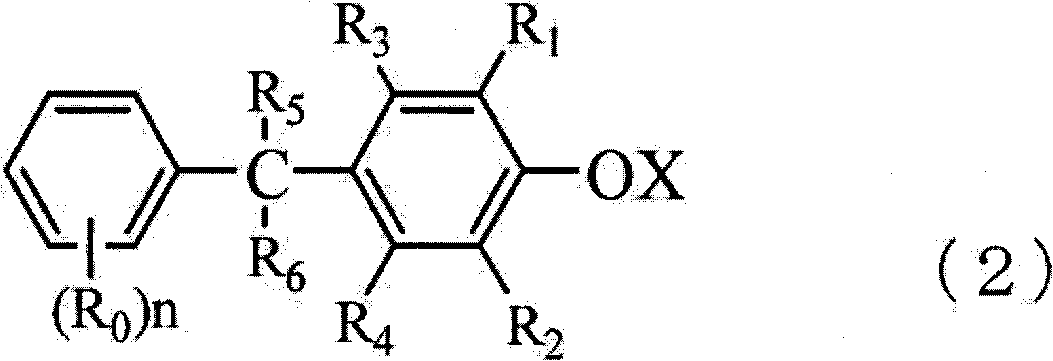
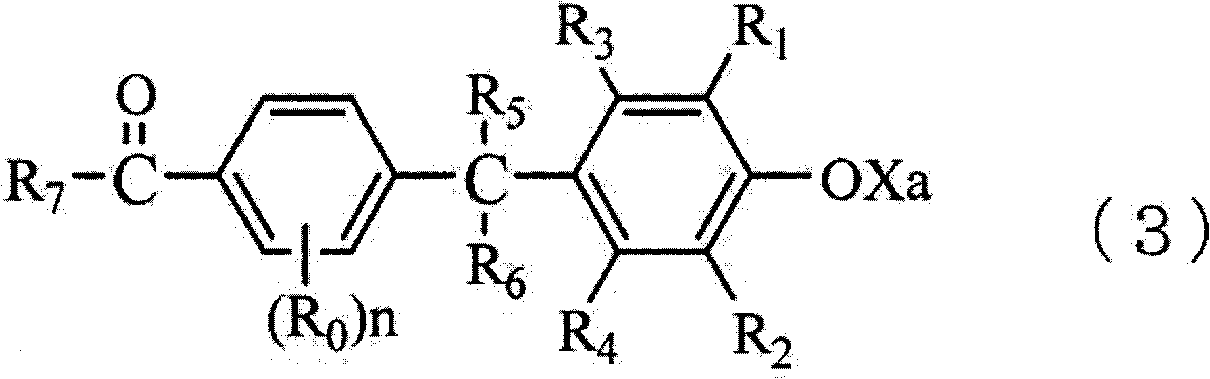
Method for producing trisphenols and monoester derivatives thereof, and 4-acylaralkylphenol derivatives
一种酰基芳烷基苯酚、制造方法的技术,应用在有机化合物的制备、羧酸酯制备、碳基化合物制备等方向,能够解决保存稳定性不佳、价格高、合成收率低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Synthesis of 4-(1-(4-acetoxyphenyl)-1-methylethyl)acetophenone (Step A1b)
[0184] 70.5 g (0.542 mol) of aluminum chloride and 105.8 g of chloroform (1.5 times the weight of aluminum chloride) were introduced into a 500 ml four-necked flask equipped with a dropping funnel, a cooling tube, and a stirrer, while replacing the inside of the system with nitrogen. Cool to 5 °C. After cooling, 42.3 g (0.542 mol) of acetyl chloride was dropped over 1 hour through the dropping funnel to form a complex. The complex will not dissolve in chloroform at 5°C, and the system will become a slurry solution.
[0185] After the complex was formed, in this solution, while maintaining the temperature in the flask at 5°C, 50.0 g (0.236 mol) of p-cumylphenol dissolved in 75 g of chloroform (relative to p-cumyl phenol) was added dropwise over 3 hours. 1.5 times the weight of cumyl phenol), after the dropwise addition was completed, it was reacted at 20°C for 2 hours.
[0186] After completio...
Embodiment 2
[0194] Synthesis of 4-(1-(4-hydroxyphenyl)-1-methylethyl)acetophenone (step C1)
[0195] 20.1 g of crystals obtained in Example 1 were dissolved in 20 g of toluene, 24.0 g of 16% aqueous sodium hydroxide solution and 2 g of methanol were added, and hydrolysis reaction was carried out at 50° C. for 2.5 hours. After the reaction, it was neutralized with 75% phosphoric acid, and then the water layer was removed.
[0196] From the obtained oil layer, toluene was distilled off at 60° C. to 10 kPa, and 18.0 g of an orange solid having a purity of 99.9% as measured by HPLC was obtained.
[0197] This solid was analyzed by NMR and mass spectrometry, and it was confirmed to be 4-(1-(4-hydroxyphenyl)-1-methylethyl)acetophenone.
[0198] Also, the yield relative to p-cumylphenol was 60.0%.
[0199] 1 H-NMR (400MHz, CDCl 3 , standard substance: tetramethylsilane)
[0200] 7.86 (aromatic H, 2H, doublet, J=8.78Hz, b in the figure), 7.32 (aromatic H, 2H, doublet, J=8.78Hz, c in the figu...
Embodiment 3
[0203] Synthesis of 1-(α,α-bis(4-hydroxyphenyl)ethyl)-4-(α-methyl-α-(4-hydroxyphenyl)ethyl)benzene (Step B1)
[0204] Into a 300 ml 4-necked flask equipped with a dropping funnel, a cooling tube, and a stirrer, 55.6 g of phenol, 1.7 g of toluene (3 wt. mol%), while replacing the inside of the system with nitrogen, the temperature was raised to 40°C.
[0205] After replacing with nitrogen gas, the inside of the system was replaced with hydrogen chloride gas. While maintaining the internal temperature of the flask at 40 to 45° C. while continuously supplying hydrogen chloride gas, the 4-[1-methyl-1-(4-hydroxyphenyl)ethane obtained in Example 2 was added dropwise over 3 hours. Base] Acetophenone 24.6g (0.096mol) dissolved in phenol 24.6g solution.
[0206] After completion of the dropwise addition, the mixture was stirred at 40° C. for 18 hours to continue the reaction. After completion of the reaction, 35.7 g of toluene was added to the reaction-completed mixture, then a 16% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com