Preparation method of acetyl levocarnitine hydrochloride, and drug application of acetyl levocarnitine hydrochloride
A technology of levocarnitine base and hydrochloride, which is applied in the field of preparation of acetyl levocarnitine hydrochloride, can solve the problems of affecting the quality of recrystallization products, large consumption of glacial acetic acid, long heating time, etc. Low, reaction-safe, and easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
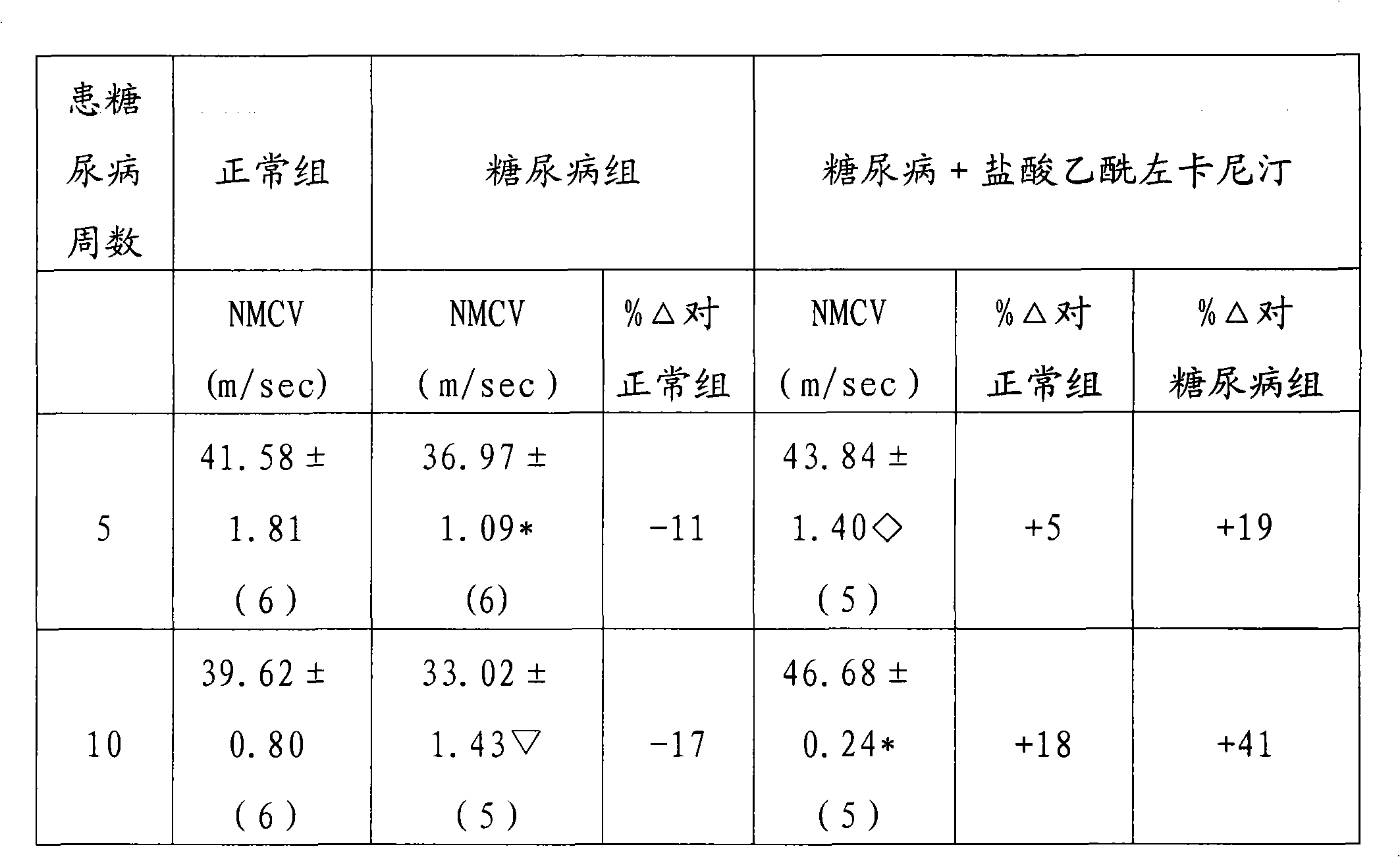
Image
Examples
preparation example Construction
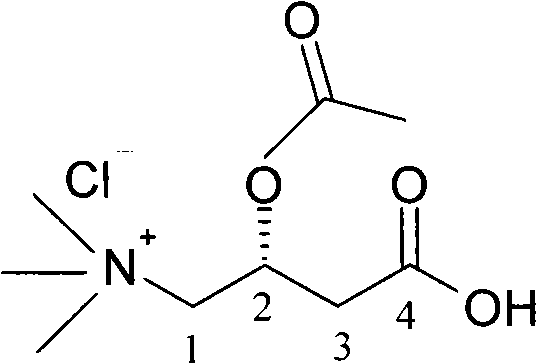
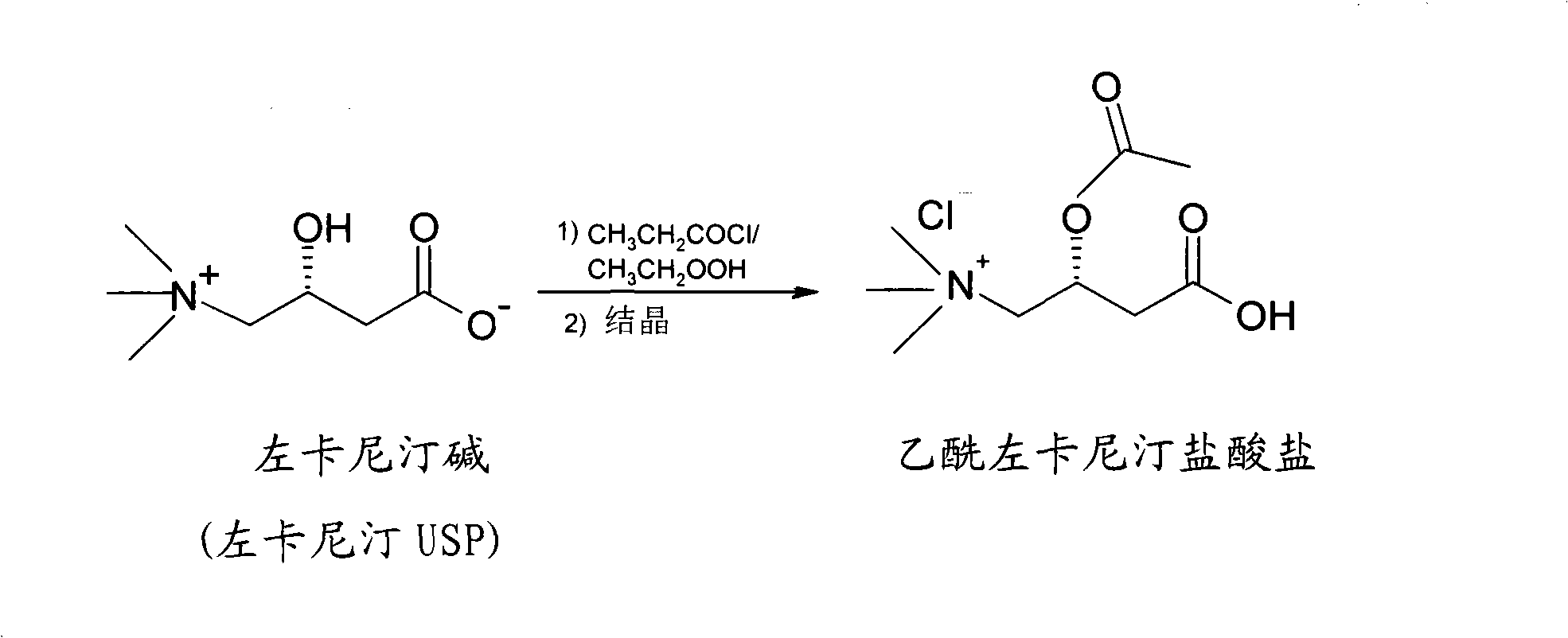
[0028] The preparation method reaction principle of acetyl-levocarnitine hydrochloride of the present invention is as follows:
[0029] Use acetyl chloride to acylate the internal salt of levocarnitine dissolved in acetic acid, add ethyl acetate, acetyl levocarnitine hydrochloride crystallizes out from the solution, and obtain purified acetyl levocarnitine after centrifugal drying Hydrochloride. The chemical reaction formula is as follows:
[0030]
Embodiment 1
[0033] (1) Preparation of acetic acid solution of levocarnitine base: adopt a 3000-liter corrosion-resistant reactor with a constant temperature jacket, such as an enamel reactor, and cover the reaction unit with nitrogen. Add 800kg acetic acid in the reactor, then add 960kg levocarnitine base in the reactor, start to stir the reactor. The temperature inside the reactor was set at 40° C., and the suspension was maintained until it was completely dissolved.
[0034] (2) Preparation of acylating reagent: use a 6000-liter corrosion-resistant reactor with a constant temperature jacket, such as an enamel reactor, and cover the reaction unit with nitrogen. Add 600 kg of acetyl chloride and 200 kg of hydrochloric acid, start stirring, and cool the reactor to below 30° C. through the cooling jacket.
[0035] (3) Acylation reaction: slowly transfer the acetic acid solution of levocarnitine base to the reactor containing the acylating reagent. Due to the exothermic reaction, the tempe...
Embodiment 2
[0041] (1) Preparation of acetic acid solution of levocarnitine base: adopt a 2500-liter corrosion-resistant reactor with a constant temperature jacket, such as an enamel reactor, and cover the reaction unit with nitrogen. Add 600kg acetic acid in the reactor, then add 540kg levocarnitine base in the reactor, start to stir the reactor. The internal temperature of the reactor was set at 42° C., and the suspension was maintained until it was completely dissolved.
[0042] (2) Preparation of acylating reagent: use a 5000-liter corrosion-resistant reactor with a constant temperature jacket, such as an enamel reactor, and cover the reaction unit with nitrogen. 300 kg of acetyl chloride and 150 kg of hydrochloric acid were added, stirring was started, and the reactor was cooled to below 30° C. through the cooling jacket.
[0043] (3) Acylation reaction: slowly transfer the acetic acid solution of levocarnitine base to the reactor containing the acylating reagent. Due to the exothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com