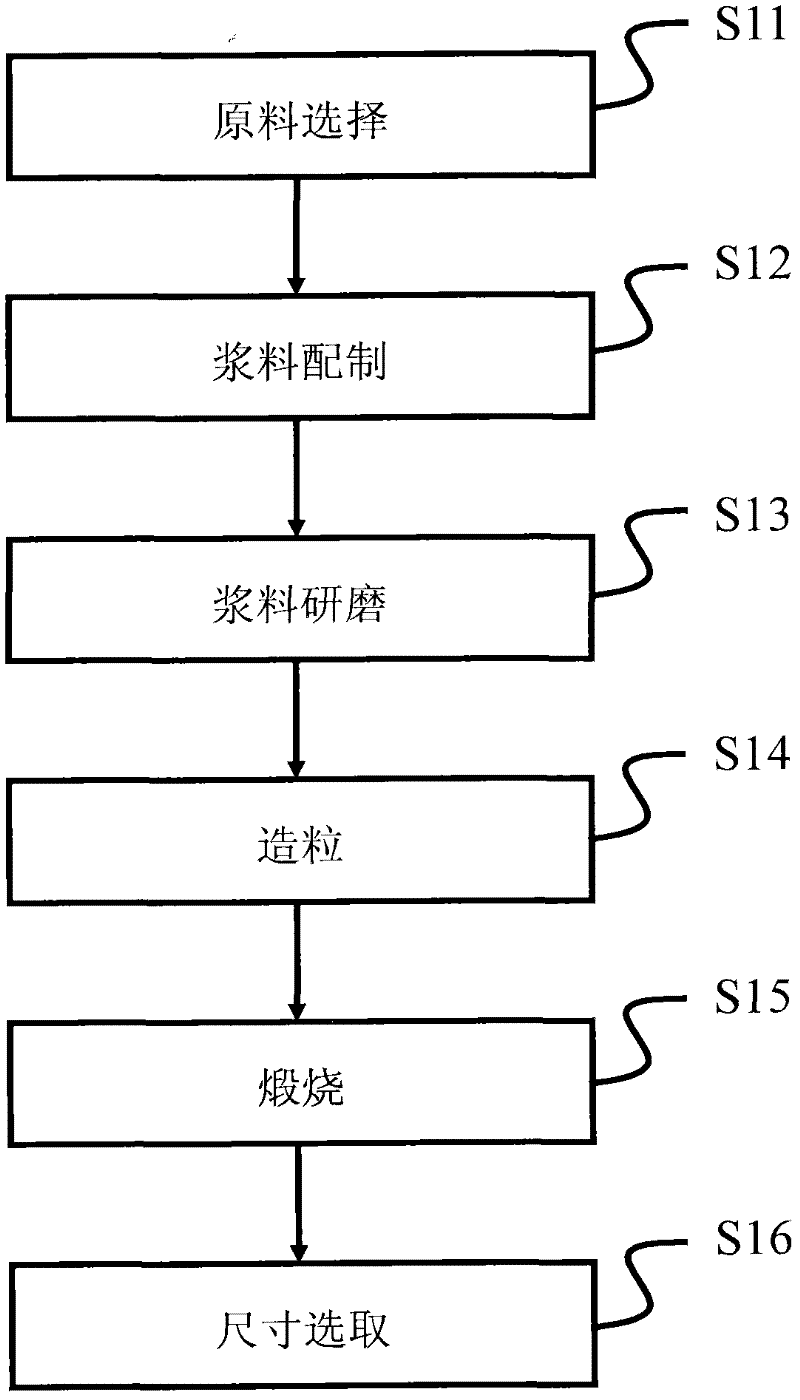
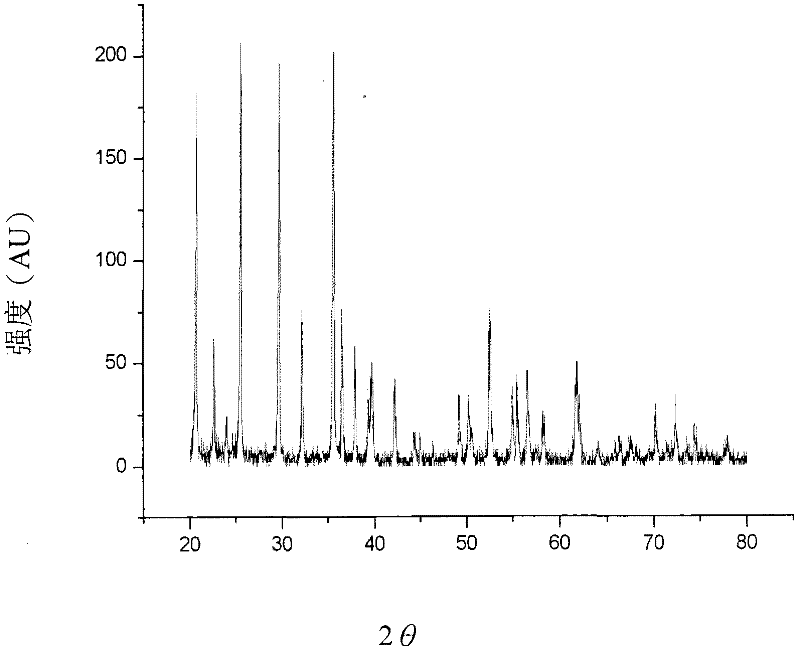
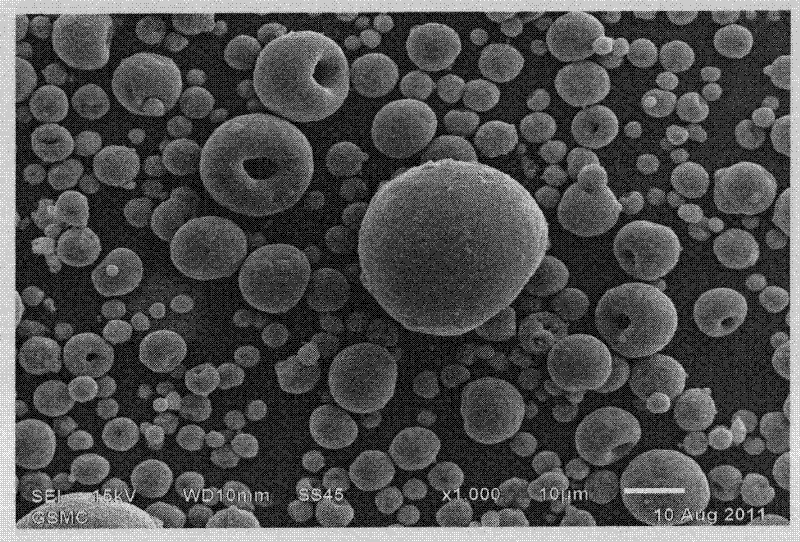
Method of preparing lithium iron phosphate material and lithium iron phosphate power prepared by the method
A technology of lithium iron phosphate and a manufacturing method, which is applied to electrical components, battery electrodes, circuits, etc., can solve the problems of small particle size of iron source materials, unfavorable slurry mixing, and difficulty in controlling the particle size of calcined powder.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] In this embodiment, ferric oxide is selected as the water-insoluble compound (iron source) and lithium dihydrogen phosphate as the water-soluble compound (phosphorus source and lithium source) in the phosphorus source, lithium source and iron source compounds, and Titanium dioxide is used as the iron site doping compound (titanium is used as the doping element replacing iron), and sucrose is used as the carbon source.
[0079] Wherein, lithium dihydrogen phosphate can be prepared as a ready-made product or in the following manner: first weigh 3.794 kg of lithium carbonate, add it to 20 kg of deionized water and stir. While stirring, 11.76 kg of 85% phosphoric acid was added to generate water-soluble lithium dihydrogen phosphate and carbon dioxide through the reaction of lithium carbonate and phosphoric acid.
[0080] In the aforementioned preparation method, because the powdered lithium carbonate is added to the aqueous solution first, and then phosphoric acid is added,...
Embodiment 2
[0089] The difference between embodiment 2 and embodiment 1 is that ferric phosphate is selected as the water-insoluble compound (phosphorus source and iron source), lithium hydroxide is used as the water-soluble compound (lithium source), niobium pentoxide is used as the lithium position doping compound, Ascorbic acid as a carbon source.
[0090] When preparing, first weigh 4.34kg of lithium hydroxide (LiOH.2H 2 O) Dissolve in 18 kg of deionized water, then add 0.28 kg of dispersant Disp 500 and 3.17 kg of ascorbic acid. After stirring, add 18.76kg of ferric phosphate and 0.336kg of niobium pentoxide. Then, as in Example 1, processes such as grinding and mixing, spray granulation, reducing atmosphere calcination, and airflow classification are carried out to produce carbon-coated lithium iron phosphate powder containing niobium, wherein the condition of reducing atmosphere calcination is sintering at 700 ° C for 16 Hour.
[0091] After measurement, the 0.2C discharge capac...
Embodiment 3
[0093] The difference between embodiment 3 and embodiment 1 is that lithium phosphate is selected as the water-insoluble lithium source and phosphorus source, and ferric nitrate (Fe(NO 3 ) 3 .9H 2 O) as a water-soluble iron source, chromium nitrate (Cr(NO 3 ) 3 .9H 2 O) as iron site doping compound (with trivalent chromium as the doping element replacing iron), glucose as carbon source.
[0094] When preparing, first weigh 38.574kg of Fe(NO 3 ) 3 .9H 2 O was dissolved in 16.5kg of deionized water, and then 3.35kg of glucose and 0.32kg of dispersant 1221 were added. After stirring, add 3.978kg of lithium phosphate and 2.01kg of Cr(NO 3 ) 3 .9H 2 O. Then, as in Example 1, the processes of grinding and mixing, spray granulation, reducing atmosphere calcination, and airflow classification are carried out to produce chromium-containing carbon-coated lithium iron phosphate powder. The condition of reducing atmosphere calcination is sintering at 720 ° C for 8 Hour.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com