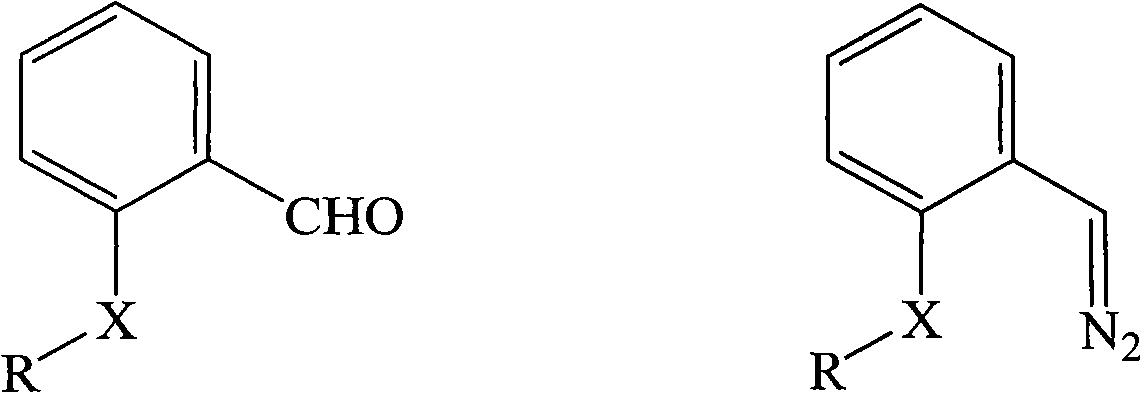Olefin metathesis catalyst and preparation method thereof
A metathesis catalyst and olefin technology, applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of complex operation, difficult preparation, high cost, etc., and achieve the effect of simple synthesis and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
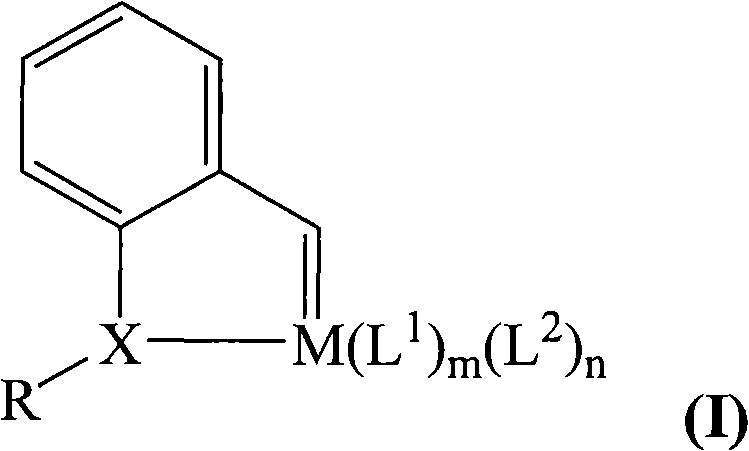
[0032] In the preparation method of the catalyst of the present invention, the reaction in step (1) can be carried out in the presence of an organic solvent. The organic solvent used may be alcohol, ether or a mixture thereof, such as methanol, ethanol, propanol, butanol, ether, tetrahydrofuran, etc. or a mixture thereof; preferably methanol, ethanol or a mixture thereof. The amount of solvent used is sufficient to ensure that the reactants are fully dissolved or dispersed.
[0033] The reaction of step (1) can be carried out in the presence or absence of a catalyst. Possible catalysts are acid compounds, such as acetic acid, p-toluenesulfonic acid, etc.; strong water absorption catalysts such as molecular sieves, titanium tetrachloride, etc. can also be used.
[0034] In step (1), the molar ratio of compound (a) to hydrazine hydrate is preferably 1:0.5-1:10, more preferably 1:1-1:5, most preferably 1:1-1:3.
[0035] The reaction in step (1) can be carried out at 10-30°C, pr...
Embodiment 1
[0050] A: Catalyst C 1 preparation of
[0051] Add o-methoxybenzaldehyde (1.36 g, 10 mmol) and hydrazine hydrate (1.0 g, 20 mmol) into a round bottom flask, add 20 mL of ethanol, and stir at room temperature for 1 hour. The reaction solution was extracted with n-pentane, the extracted organic phase was dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain oily ①-diazo compound (0.91 g, 6.1 mmol, yield 61%).
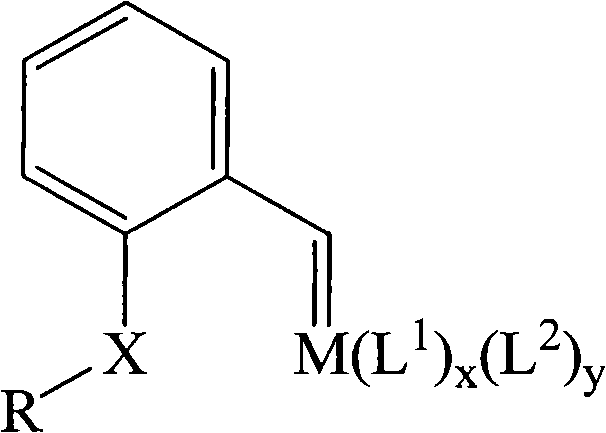
[0052] The above oil ① (0.3g, 2mmol) was dissolved in 5mL of n-pentane, cooled in an ice bath, and added into 10mL of dichloromethane dissolved with tris(triphenylphosphine)ruthenium dichloride (0.95g, 1mmol), The mixture was cooled to -78°C and reacted for 0.5 hour with stirring. The solvent was removed by rotary evaporation, and the residue was recrystallized from dichloromethane / n-pentane to obtain a khaki solid ② (0.80 g, 0.98 mmol, yield 98%). Its elemental analysis data is C 44 h 38 Cl 2 OP 2 Ru (found): C, 64.71 ...
Embodiment 2
[0057] Example 2 Catalyst C 2 preparation of
[0058] The preparation method is the same as in Example 1, but o-methoxybenzaldehyde is replaced by o-ethoxybenzaldehyde. Catalyst C 2 The elemental analysis data for C 27 h 25 Cl 2 OPRu (found): C, 57.05 (57.11); H, 4.43 (4.55).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com