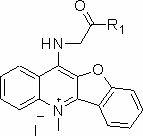
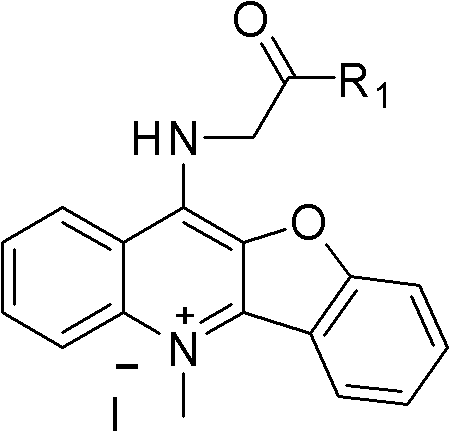
Peptide derivative of benzfuran quinoline and preparation method thereof and application thereof as antitumor medicament
A technology of benzofuran quinoline and derivatives, which is applied in the preparation of anticancer drugs. The field of peptide derivatives of benzofuran quinoline and its preparation can solve the problems of application restrictions and limited resources. Achieve high safety, broad market prospects, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
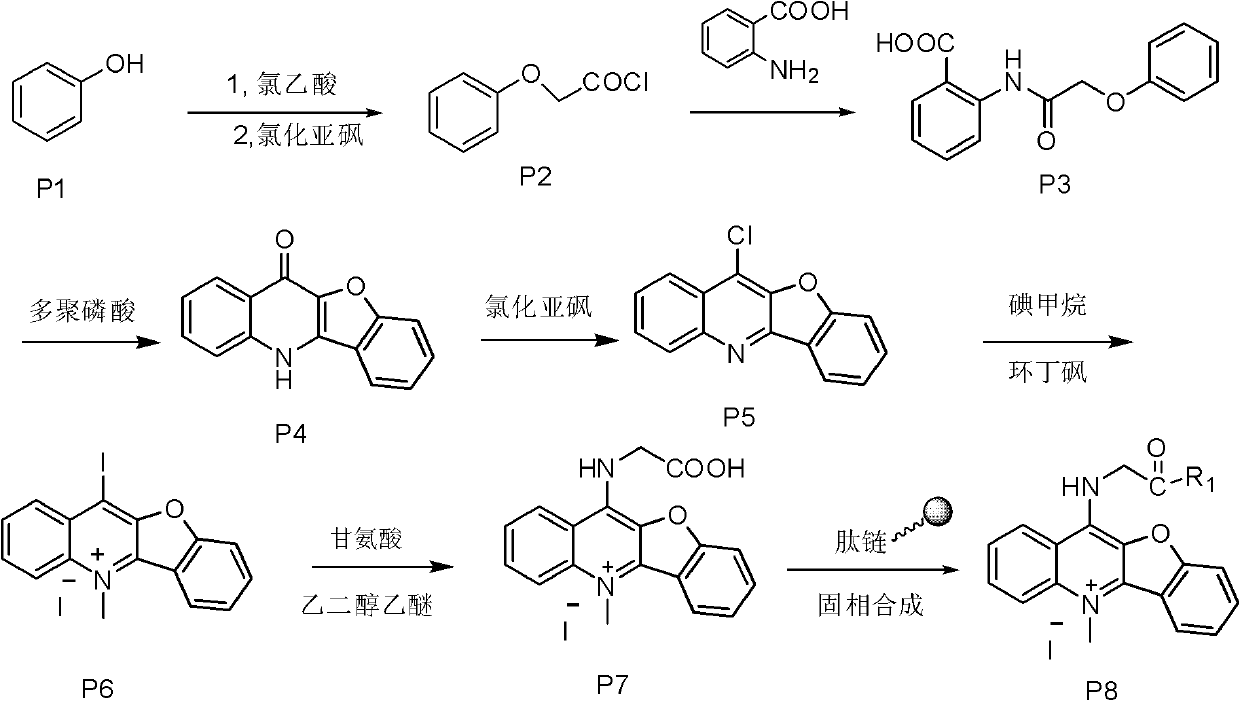
[0026] Embodiment one: the synthesis of compound P7
[0027] Dissolve 0.3mol of chloroacetic acid in 60ml of water, adjust the pH to 9 with sodium hydroxide, then add 0.2mol of phenol, reflux at 100°C, and then add thionyl chloride for chlorination reaction to obtain P2, evaporate the thionyl chloride The solvent was used to obtain a brown liquid, which was then condensed with anthranilic acid to obtain P3, and then PPA was preheated to 130°C and added to P3 for a compound reaction to obtain compound P4. Chlorination reaction of P4 with thionyl chloride at 80°C under reflux to obtain compound P5, and methylation reaction with methyl iodide in a system of sulfolane as a solvent to obtain compound P6. Then 0.05 mol P6 and 0.25 mol glycine were refluxed at 120°C for 4 h in ethylene glycol ether as a solvent, and purified by silica gel chromatography with methanol / dichloromethane as eluent to finally obtain light green compound P7.
[0028] Yield: 41%; 1 H NMR (400MHz, DMSO) δ9....
Embodiment 2
[0031] Embodiment two: the synthesis of compound R
[0032] Dissolve 0.2mol Fmoc-Arg(Pbf)-OH amino acid in DMF (dimethylformamide) solvent, add 0.8mol HOBT (1-hydroxybenzotriazole), 0.8mol DIC (N,N-diisopropyl Carbodiimide) two condensation reagents (condensation reagent ratio is 1: 1), in solid phase reactor with deprotected Rink Amide AM resin reaction 3h, then remove Fmoc group with 25% piperidine, Get amino acid side chains. Then it was condensed with P7 in DMF solution of HOBT and DIC. After 24 hours, the resin was removed with trifluoroacetic acid, collected, and purified by preparative high-efficiency chromatography to finally obtain light yellow solid R.
[0033] Yield: 32%; 1H NMR (400MHz, DMSO) δ9.71(t, J=6.4Hz, 1H), 8.71(d, J=8.5Hz, 1H), 8.63(d, J=8.2Hz, 1H), 8.56(d, J =8.2Hz, 1H), 8.43(d, J=9.0Hz, 1H), 8.15-8.07(m, 1H), 7.87(ddd, J=21.5, 15.3, 8.0Hz, 3H), 7.64(t, J= 7.4Hz, 2H), 7.49(s, 1H), 7.13(s, 2H), 4.79(d, J=6.4Hz, 2H), 4.58(s, 3H), 4.25(dd, J=14.7, 7.0Hz,...
Embodiment 3
[0036] Embodiment three: the synthesis of compound GG
[0037] The method is the same as in Example 2, except that Fmoc-Gly-OH is used, and the amino acid is connected to the Rink Amide AM resin twice. Finally, after purification by preparative high performance chromatography, GG was finally obtained as a white solid.
[0038] Yield: 29%; 1 H NMR (400MHz, DMSO) δ9.72(t, J=6.5Hz, 1H), 8.70(t, J=8.4Hz, 2H), 8.64(d, J=8.2Hz, 1H), 8.44(d, J =8.9Hz, 1H), 8.12(dd, J=9.8, 6.4Hz, 2H), 7.93(t, J=5.9Hz, 2H), 7.87-7.77(m, 1H), 7.65(ddd, J=8.2, 5.8, 2.4Hz, 1H), 7.23(s, 1H), 7.02(s, 1H), 4.78(d, J=6.4Hz, 2H), 4.59(s, 3H), 3.83(d, J=5.7Hz, 2H), 3.60(d, J=5.8Hz, 2H); C 22 h 22 N 5 o 4 + , LC-MS m / z: 420[M+H] + .
[0039]
[0040] Compound GG
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com