Tetrahymena expression vector of chitinases and its application in expressing chitinases
A technology of chitinase and expression vector, which is applied in the application field of expressing chitinase, can solve problems such as difficult to achieve high fermentation density, easy protein degradation, inactivity, etc., and achieve operational accuracy and controllability Strong, wide application prospects, short life cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the construction of recombinant expression vector
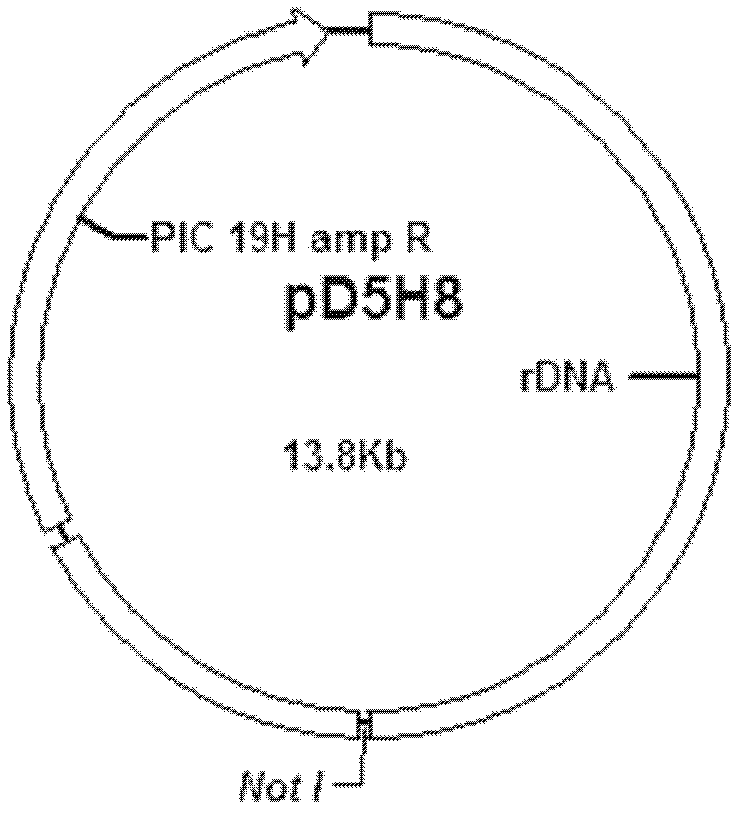
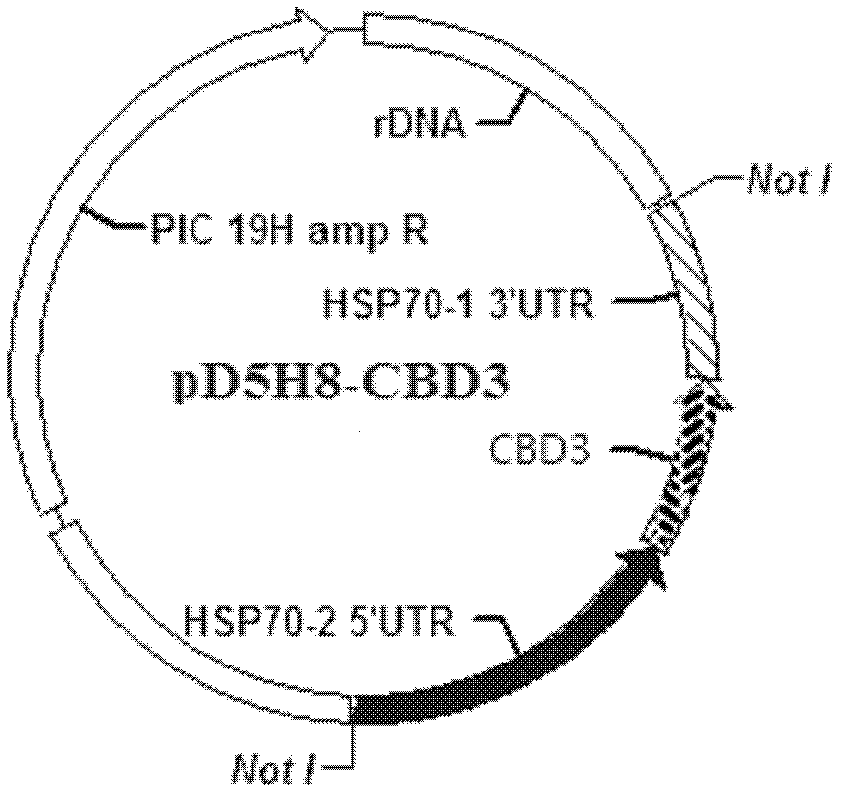
[0037] 1. Prepare the DNA fragment (4312bp) shown in Sequence 1 of the sequence listing, digest it with restriction endonuclease Not I and recover the digested product.
[0038] In the DNA fragment shown in Sequence 1 of the sequence listing, the 1st to 8th nucleotides from the 5' end are the Not I restriction recognition sequence, and the 9th to 1126th nucleotides are the HSP70-2 gene promoter (HSP70- 25'UTR), the 1142nd to 3820th nucleotide is the coding gene of chitinase (CBD3 gene), the 3835th to 4304th nucleotide is the HSP70-1 gene termination signal (HSP70-13'UTR), the 1st Nucleotides 4305 to 4312 are the recognition sequence for Not I digestion.
[0039] The DNA fragment shown in Sequence 1 of the Sequence Listing can be artificially synthesized.
[0040] The DNA fragment shown in Sequence 1 of the sequence listing can also be prepared as follows:
[0041] (1) Construction of recombinant plasmi...
Embodiment 2
[0059] Embodiment 2, utilizing Tetrahymena to express chitinase
[0060] 1. Transfection of recombinant plasmid pD5H8-CBD3 into Tetrahymena
[0061] 1. Starvation treatment of Tetrahymena
[0062] (1) Transfer Tetrahymena thermophila (Tetrahymena thermophila B2086 strain or CU428 strain) to 50ml SPP medium (in a 250ml Erlenmeyer flask), and cultivate it at 30°C on a shaker at 160rpm to Cell density reaches 3-5 x 10 5 Individuals / ml (about 18 hours).
[0063] (2) Transfer the culture system (50ml) of step (1) into a 50ml centrifuge tube, centrifuge at 1000g for 2min at 30°C, collect the precipitate (Tetrahymena), and wash twice with 50ml Tris buffer incubated at 30°C.
[0064] (3) Add a small amount of Tris buffer incubated at 30°C to a 50ml centrifuge tube (including the precipitate in step (2), shake gently to remove the precipitate, and then transfer all the liquid in the tube and Tetrahymena to a sterile conical tube In the flask, adjust the cell density to 3×10 with Tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com