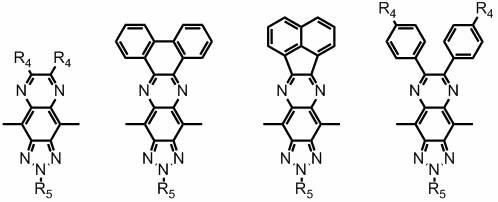
Pyrazine-ring-containing organic dyes and preparation method and use thereof
A technology of organic dyes and pyrazine rings, applied in organic dyes, chemical instruments and methods, methenyl/polymethenyl dyes, etc., can solve problems such as large-scale industrialization restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Dye intermediate 1 preparation of c
[0061] Under nitrogen protection, 1.00 g (2.39 mmol) 1 a (References for synthetic methods Mondal, R.; Miyaki, N.; Becerril, H. A.; Norton, J. E.; Parmer, J.; Mayer, A. C.; Tang, M. L.; Bredas, J. L.; McGehee, M. D.; Bao, Z. N. Chem. Mater. 2009 , 21 , 3618.), 2.43 g (5.00 mmol) 1 b Dissolve in 40 mL DMF, add 110 mg (0.10 mmol) Pd(PPh 3 ) 4 , heated to 85°C, reacted for 5 hours, when the reaction was complete, the reaction system was poured into water, extracted with dichloromethane, the organic phase was washed with water for several times, dried, the solvent was removed, column separation and purification obtained 1.33 g of compound 1c , yield 86%.
Embodiment 2
[0062] Embodiment 2: dye intermediate 1 preparation of d
[0063] Under nitrogen protection, 0.80 g (1.23 mmol) 1 c was dissolved in 30 mL of dichloromethane, added 0.12 mL (1.29 mmol) of phosphorus oxychloride, 0.14 mL (1.81 mmol) of DMF, heated to 70 ° C and refluxed for 8 hours, after the reaction was completed, added saturated sodium acetate and stirred for half The pH of the solution was adjusted to 7 with sodium hydroxide solution. It was extracted with dichloromethane, the organic phase was washed several times with water, dried, the solvent was removed, and 0.78 g of compound 1d was obtained by column separation and purification, with a yield of 93%.
Embodiment 3
[0064] Embodiment 3: dye intermediate 1 preparation of e
[0065] Under nitrogen protection, 0.74 g (1.09 mmol) 1 d was dissolved in 30 mL chloroform and 10 mL glacial acetic acid, added 0.29 g (1.64 mmol) N-bromosuccinimide, stirred at room temperature for 5 hours, after the reaction was completed, water was added to terminate the reaction, extracted with dichloromethane, organic The phase was washed several times with sodium hydroxide solution and water, dried, and the solvent was removed, and purified by column separation to obtain 0.69 g of compound 1e with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com