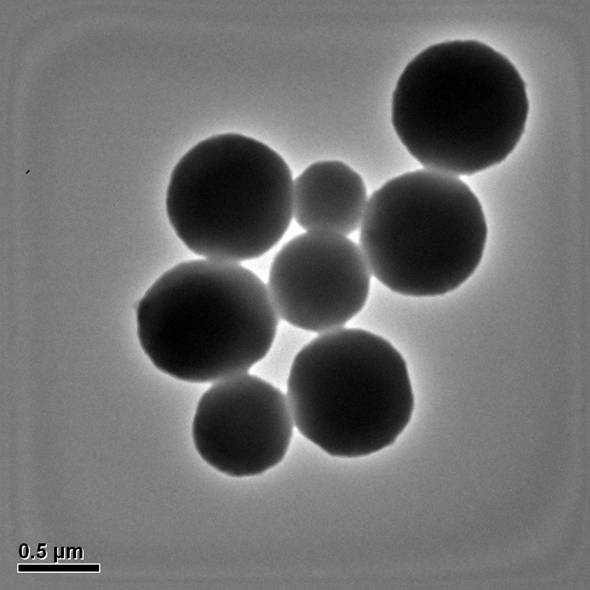
Method for preparing porous carbon spheres doped with nitrogen and phosphorus and application
A phosphorus-doped, porous carbon technology, applied in chemical instruments and methods, carbon compounds, inorganic chemistry, etc., can solve the problems of complex preparation process of porous carbon materials, environmental pollution, etc., to achieve hydrogen storage and environmental pollution Small, easily batch-preparable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) In a 150 mL dry flask, add 100 mL of acetonitrile, weigh 0.001 mol of hexachlorocyclotriphosphazene and 0.003 mol of 4,4′-dihydroxydiphenyl sulfone, mix them evenly and add 0.01 mol of three Ethylamine, seal the flask immediately, and react in an ultrasonic cleaner (100W, 40kHz) water bath for 2h at 40°C. Centrifuge after the reaction, and wash the centrifuged product with ethanol and deionized water for 3 to 4 times, and finally dry the product at 80° C. for 24 hours to obtain polyphosphazene microspheres.
[0033] (2) Weigh 0.6g of polyphosphazene microspheres and 0.6g of potassium hydroxide, disperse them in 4 mL of deionized water, stir for 2 hours to fully immerse the activator potassium hydroxide into the polyphosphazene microspheres, and then dissolve the above mixed solution Centrifuge and dry at 80°C for 24 hours to obtain a polyphosphazene microsphere / potassium hydroxide mixture.
[0034](3) Put the obtained polyphosphazene microspheres / potassium hydroxid...
Embodiment 2
[0053] (1) In a 150 mL dry flask, add 50 mL of acetone, weigh 0.001 mol of hexachlorocyclotriphosphazene and 0.0028 mol of 4,4′-diaminodiphenyl sulfone, mix them well and add 0.006 mol of three Ethylamine, seal the flask immediately, and react in an ultrasonic cleaner (100W, 40kHz) water bath for 10h at 20°C. Centrifuge after the reaction, and wash the centrifuged product with ethanol and deionized water for 3 to 4 times, and finally dry the product at 50° C. for 20 hours to obtain polyphosphazene microspheres.
[0054] (2) Weigh 0.6g of polyphosphazene microspheres and 0.3g of sodium hydroxide, disperse them in 2 mL of deionized water, stir for 2 hours to fully immerse the activator sodium hydroxide into the polyphosphazene microspheres, and then dissolve the above mixed solution Centrifuge and dry at 50°C for 20 hours to obtain a polyphosphazene microsphere / sodium hydroxide mixture.
[0055] (3) Put the obtained polyphosphazene microspheres / sodium hydroxide mixture in a por...
Embodiment 3
[0058] (1) In a 150 mL dry flask, add 500 mL of ethanol, weigh 0.001 mol of hexachlorocyclotriphosphazene and 0.0032 mol of hydroquinone, mix well, add 0.012 mol of triethylamine to it, and immediately seal the flask , react in an ultrasonic cleaner (100W, 40kHz) water bath for 1h at 80°C. Centrifuge after the reaction, and wash the centrifuged product with ethanol and deionized water for 3 to 4 times, and finally dry the product at 70° C. for 12 hours to obtain polyphosphazene microspheres.
[0059] (2) Weigh 0.6g of polyphosphazene microspheres and 3g of calcium chloride, disperse them in 6 mL of deionized water, stir for 2 hours to fully immerse the activator calcium chloride into the polyphosphazene microspheres, and then centrifuge the above mixed solution And dry at 70° C. for 12 hours to obtain a polyphosphazene microsphere / calcium chloride mixture.
[0060] (3) Put the obtained polyphosphazene microspheres / calcium chloride mixture in a porcelain boat and send it to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com