Cubic water quality liquid crystal gel transdermic absorption preparation containing oxybutynin chloride and preparing method thereof
A gel and liquid crystal technology, applied in the field of preparations containing oxybutynin hydrochloride or its base, can solve problems such as difficult mixing, poor patient compliance, drug precipitation, etc., to improve moisturizing effect, repair integrity, moisturizing skin effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
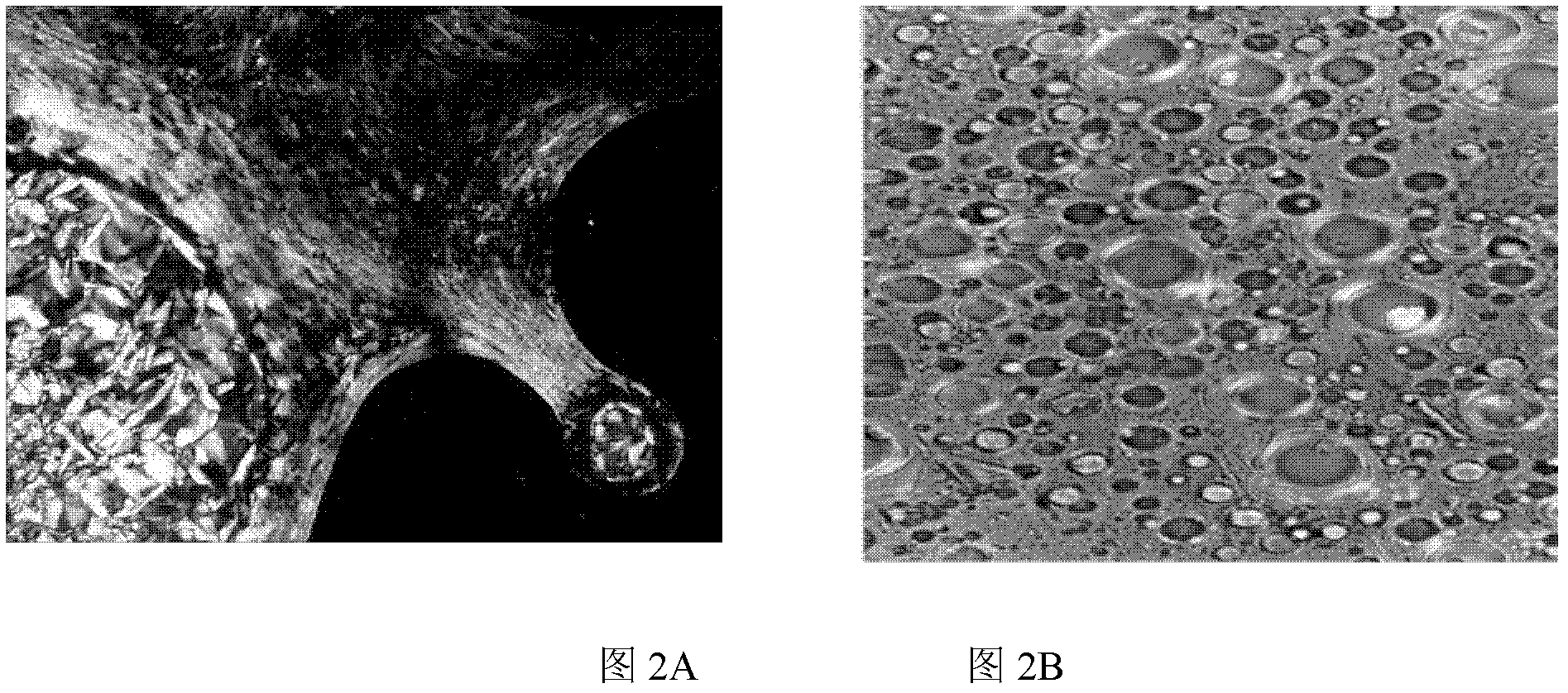
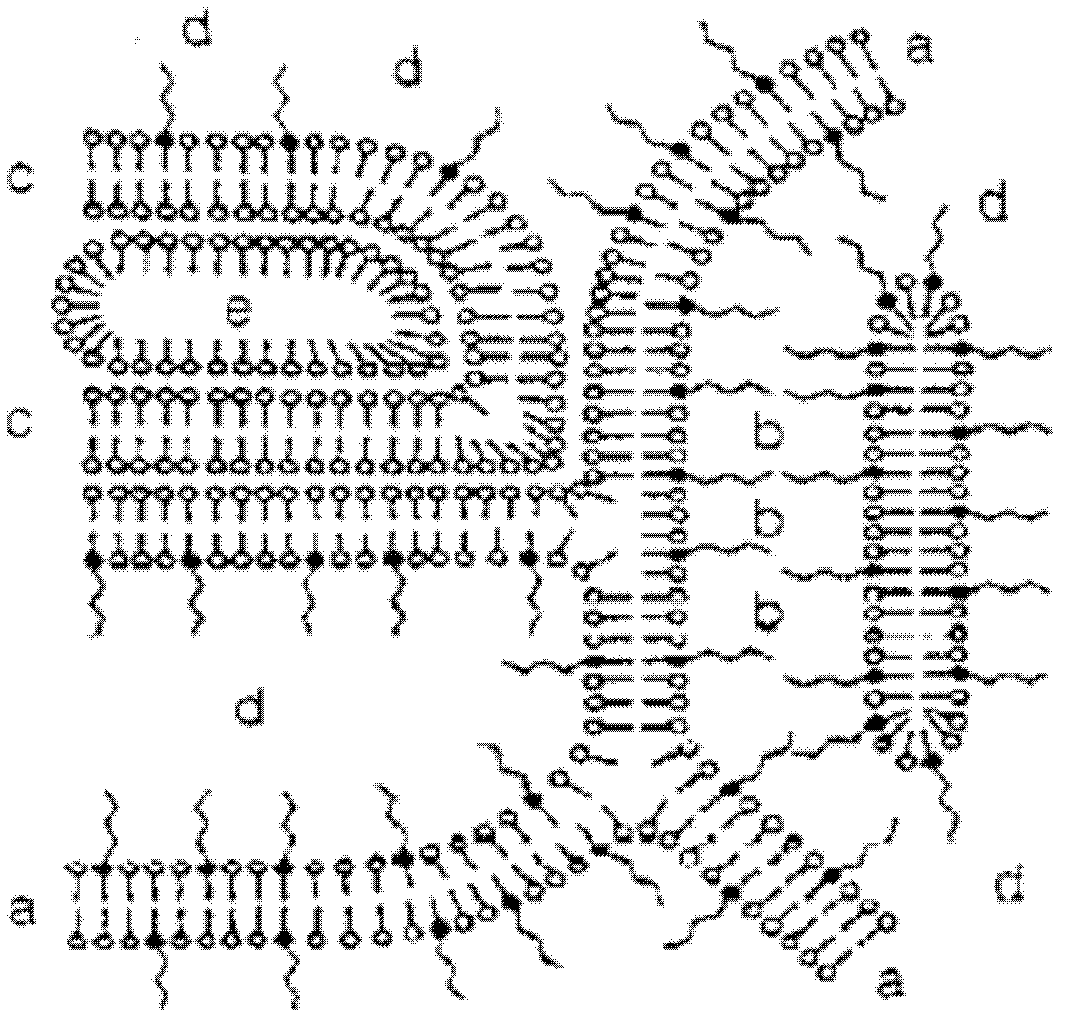
Image
Examples
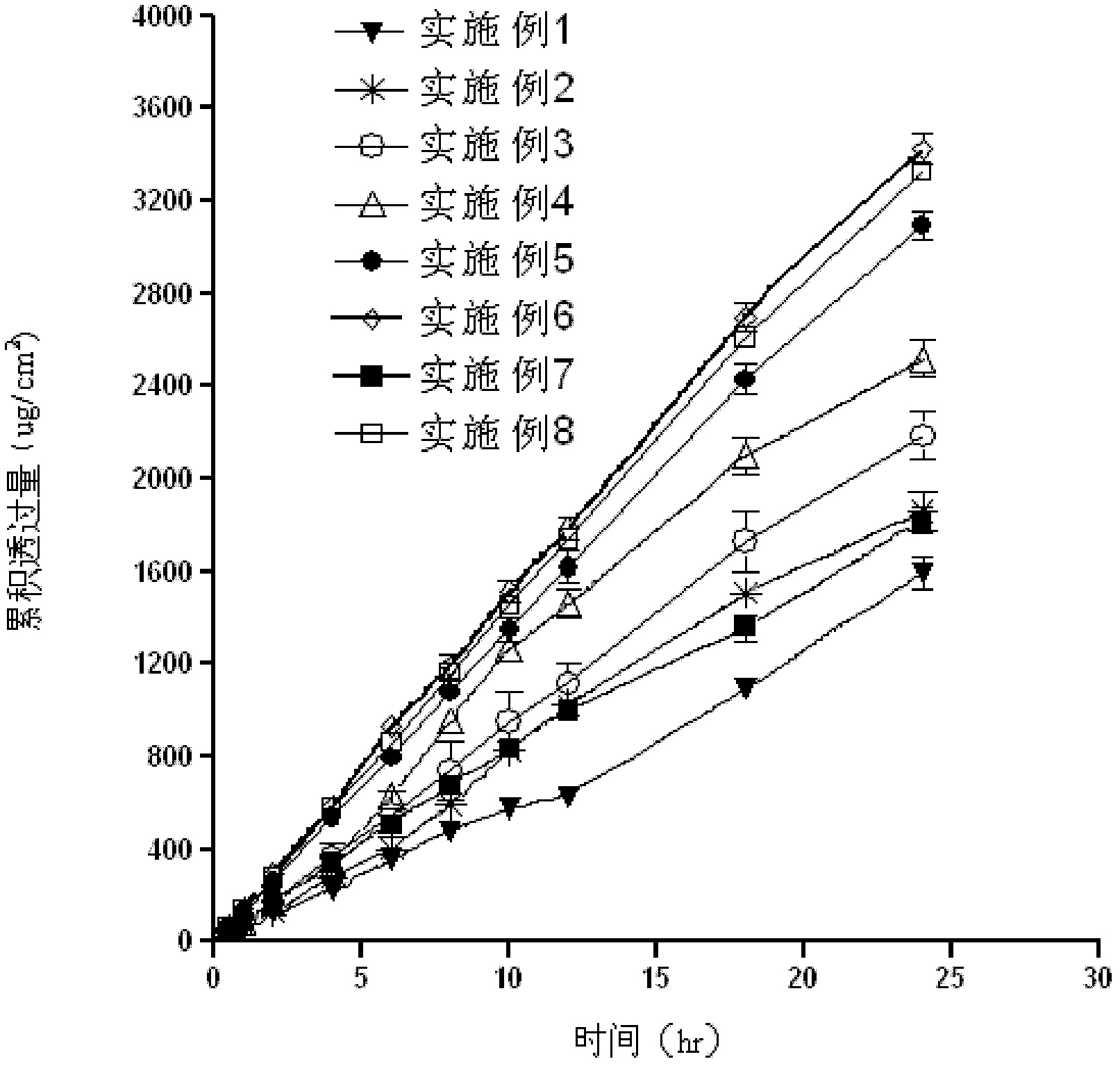
Embodiment 1~8
[0063] See Table 1 for the prescription.
[0064] Table 1
[0065]
[0066] Table 1 continued (1)
[0067]
[0068] Table 1 continued (2)
[0069]
[0070]
[0071] Giga Emulgin S-68, the product of Giga Fine Chemical Company, has an HLB value of 8;
[0072] OW-340B is a product of CRODA Ltd, with an HLB value of 12;
[0073] Arlatone LC, a product of CRODA Ltd, has an HLB value of 5.5;
[0074] Ethanol is in volume concentration.
[0075] Preparation:
Embodiment 1
[0076] The preparation method of embodiment 1:
[0077] (1) Dissolving the drug in solvent 1 and mixing to obtain the drug solution, which is phase A;
[0078] (2) The gel matrix material and antibacterial agent are mixed and swelled with solvent 2 for 24 hours to obtain a hydrogel matrix, then add oily transdermal penetration enhancer and antioxidant, stir at 500rpm for 2 hours, mix and disperse to obtain a hydrogel The carrier is phase B;
[0079] Then add phase A to phase B, stir at 500rpm for 2 hours, mix and disperse to obtain a drug-containing hydrogel;
[0080] (3) Add liquid crystal gel emulsion into water, heat and melt at 60°C to obtain phase C;
[0081] (4) Add phase C to the drug-containing hydrogel at 50°C, stir at 50 rpm for 2 hours, and self-emulsify to form a liquid crystal gel;
[0082] (5) Add a pH adjuster to the liquid crystal gel at 25° C. and stir at 50 rpm for 30 minutes to obtain the oxybutynin-containing gel percutaneous absorption preparation.
Embodiment 2
[0083] The preparation method of embodiment 2:
[0084] (1) Dissolving the drug in solvent 1 and mixing to obtain the drug solution, which is phase A;
[0085] (2) The gel matrix material and antibacterial agent are mixed and swelled with solvent 2 for 4 hours to obtain a hydrogel matrix, then add oily transdermal penetration enhancer and antioxidant, stir at 5000rpm for 0.5 hour, mix and disperse to obtain a hydrogel The carrier is phase B;
[0086] Then add phase A to phase B, stir at 5000rpm for 0.5 hours, mix and disperse, and obtain a hydrogel containing medicine;
[0087] (3) Add liquid crystal gel emulsion into water, heat and melt at 100°C to obtain phase C;
[0088] (4) At 70°C, add phase C to the drug-containing hydrogel, stir at 200rpm for 0.5 hours, and self-emulsify to form a liquid crystal gel;
[0089] (5) Add a pH adjuster to the liquid crystal gel at 35° C. and stir at 200 rpm for 10 minutes to obtain the oxybutynin-containing gel percutaneous absorption pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com