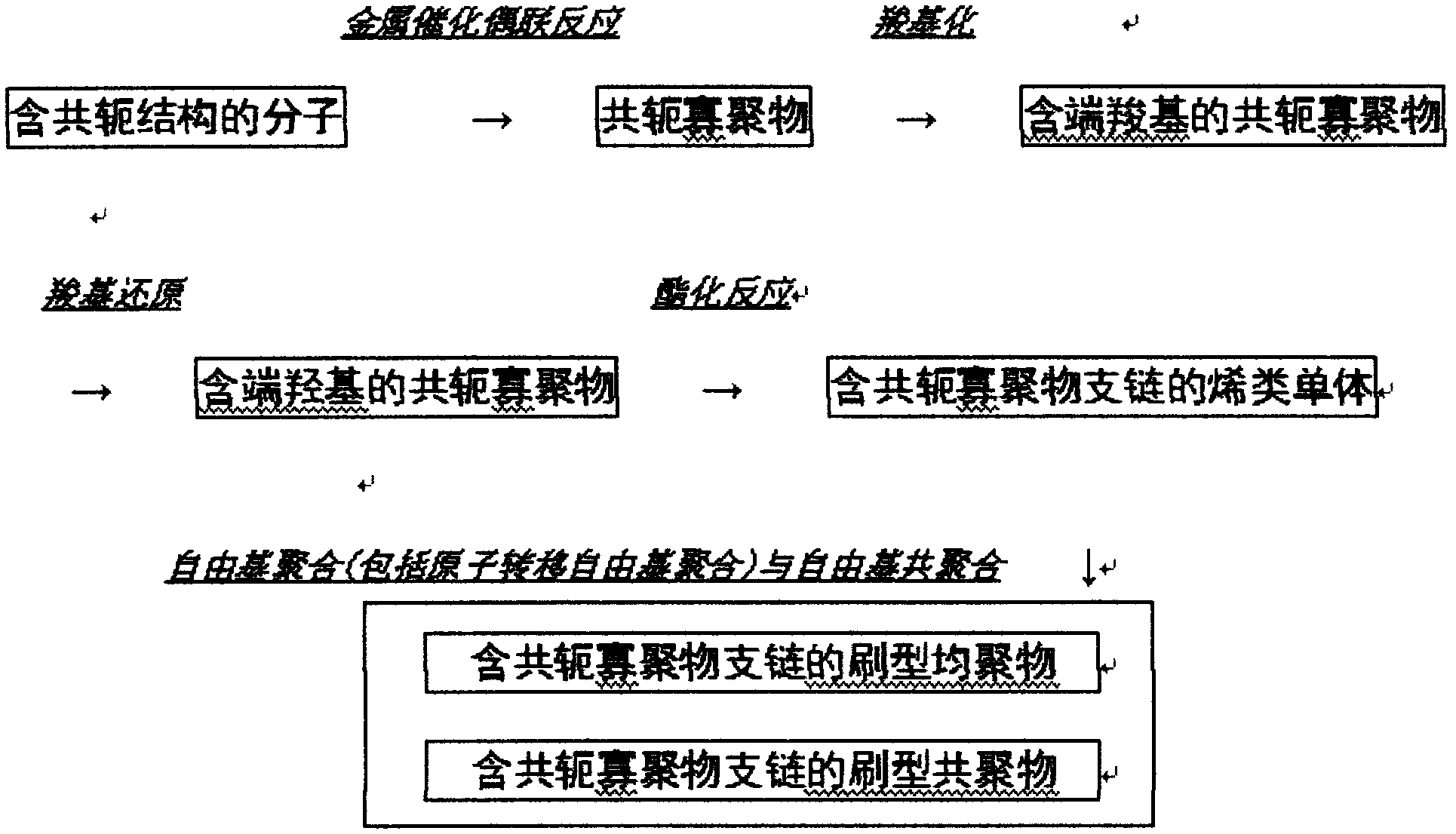
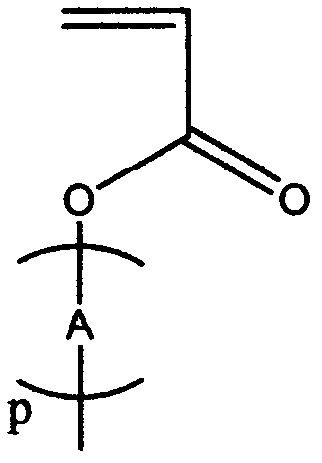
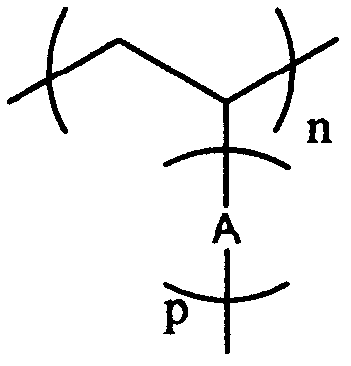
Synthetic method of brush type homopolymer and copolymer containing conjugate oligomeric branched chains
A synthesis method and oligomer technology, applied in chemical instruments and methods, preparation of organic compounds, organic silicon compounds, etc., can solve the problems of difficult molecular weight growth, poor structural regularity, harsh reaction conditions, etc., and achieve a simple, convenient and easy method. The effect of line, less defects and controllable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Synthesis of a brush-type homopolymer containing bis-fluorene branches
[0059] (1) Synthesis of bis-fluorene-containing monomers
[0060] a. Dissolve equimolar amounts of monobromo 9,9'-dibutylfluorene and monoboronic acid 9,9'-dibutylfluorene in N,N-dimethylformamide, and add molar amounts respectively as reactants 0.2% of triphenylphosphine palladium and 0.2% of the reactant molar concentration of 2mol / L sodium carbonate solution, heated to 90 ° C for 2 days, the mixture was extracted, washed with saturated brine, and then anhydrous sulfuric acid Sodium drying, the primary product obtained by removing the solvent was subjected to column chromatography to obtain a solid bis-9,9'-dibutylfluorene;
[0061] b. Dissolve the double-linked 9,9'-dibutylfluorene prepared in step a of step (1) in tetrahydrofuran, cool to minus 78°C, and dropwise add equimolar n-butyl fluorene with a molar concentration of 1.6mol / L Lithium solution, after reacting for one hour, car...
Embodiment 2
[0067] Example 2: Synthesis of brush-type binary block copolymers containing pentafluorene branched chains and bisthiophene branched chains
[0068] (1) Synthesis of pentafluorene-containing monomers and dithiophene-containing monomers
[0069] ①. Synthesis of pentafluorene-containing monomers
[0070] a. Dissolve 2,7-dibromo-9,9'-dibutylfluorene in tetrahydrofuran, cool to minus 78°C and add dropwise an equimolar amount of n-butyllithium solution with a molar concentration of 1.6mol / L to react One hour later, equimolar trimethylchlorosilane was added, and the reaction was continued for half an hour. The mixture was extracted, washed with saturated brine, and dried with anhydrous sodium sulfate. The initial product obtained by removing the solvent was obtained by column chromatography to obtain 2- Bromo-9,9'-dibutyltrimethylsilafluorene.
[0071] b. Dissolve the 2-bromo-9,9'-dibutyltrimethylsilafluorene prepared in step (1) step a in tetrahydrofuran, cool to minus 78°C and d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com