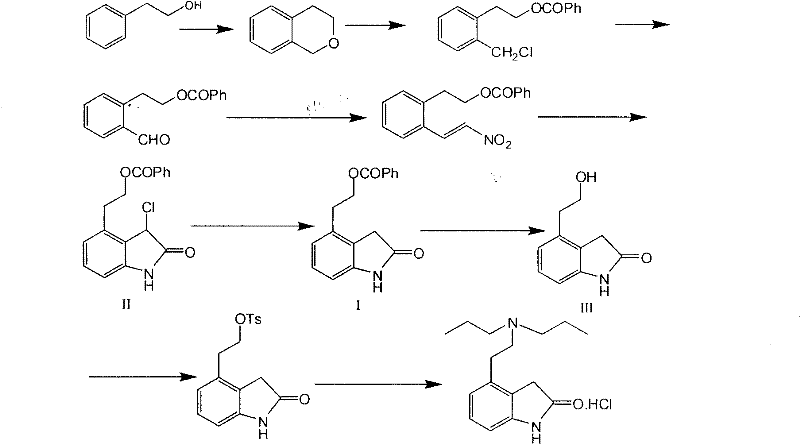
Novel method for preparing ropinirole hydrochloride intermediate 4-(beta-ethoxyl)-1,3-dihydro-2H-indolyl-2-ketone
A hydrogen donor and inert gas technology, applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, low reaction yield, long production cycle, etc., and achieve the effects of mild reaction conditions, high product yield and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
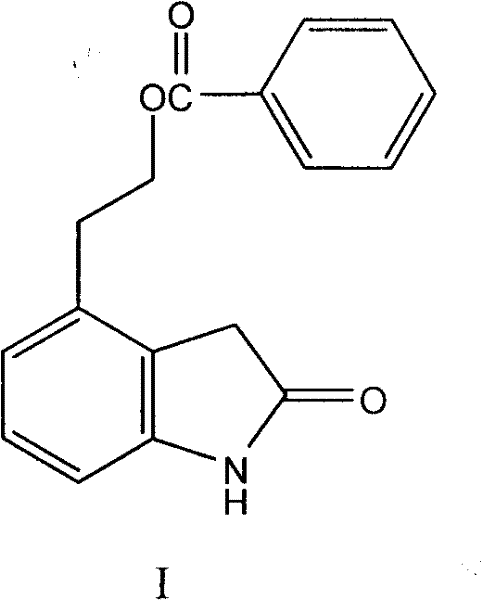
[0021] Embodiment 1, the preparation of compound I
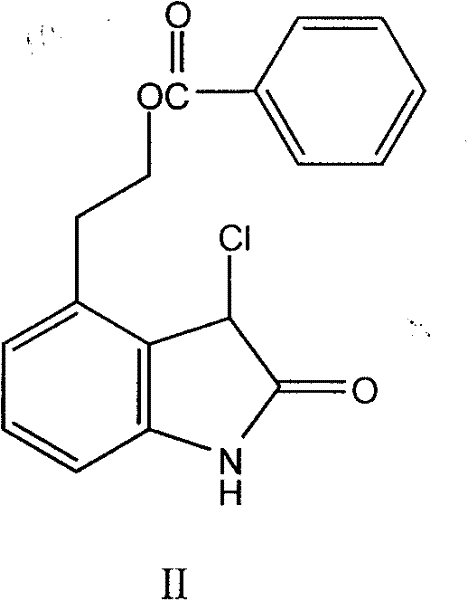
[0022] Nitrogen was passed into the reaction flask, compound II 44.0g, palladium hydroxide 8.8g, methanol 835ml were added successively, the temperature was raised to 40-50°C, 17.6g of ammonium formate aqueous solution was added dropwise (use 220ml of water to dissolve ammonium formate), and the dropwise , heat preservation reaction for 30 to 40 minutes, after the reaction is completed, suction filter while it is hot, wash the filter cake with water and dichloromethane successively, collect the filtrate, separate the liquids, extract the water layer with dichloromethane, and evaporate the dichloromethane layer to dryness under reduced pressure to obtain Off-white solid 37.3g, purity 97.8%.
Embodiment 2
[0023] Embodiment 2, the preparation of compound I
[0024] Feed nitrogen into the reaction flask, sequentially add 35.0g of compound II, 17.5g of 5% palladium carbon, and 700ml of methanol, heat up to reflux, add dropwise 17.0g of 90% aqueous solution of formic acid, dropwise, keep warm for 30 to 40 minutes, and react After completion, suction filter while hot, wash the filter cake with water and dichloromethane successively, collect the filtrate, separate the liquids, extract the water layer with dichloromethane, and evaporate the dichloromethane layer to dryness under reduced pressure to obtain 26.1 g of off-white solid with a purity of 96.1%. .
Embodiment 3
[0025] Embodiment 3, the preparation of compound I
[0026] Introduce nitrogen into the reaction flask, add 35.0g of compound II, 5.0g of Raney nickel, 700ml of ethanol in sequence, raise the temperature to reflux, add dropwise 17.0g of 90% aqueous solution of formic acid, dropwise, keep warm for 30-40 minutes, and the reaction is complete , filtered while hot, washed the filter cake with water and dichloromethane successively, collected the filtrate, separated the liquids, extracted the water layer with dichloromethane, and evaporated the dichloromethane layer to dryness under reduced pressure to obtain 27.3 g of an off-white solid with a purity of 96.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com