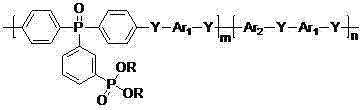Novel polymer containing phosphonate group and preparation method and application of novel polymer
A phosphonate group and polymer technology, which is used in the synthesis of polyaryl ether phosphine oxide polymers and their preparation, as well as in the field of new polymer materials, can solve weak resistance, increase fresh water treatment process technology, easy to shrink deformation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of bis(4-fluorophenyl)(3'-diethoxyphosphonophenyl)phosphine oxide
[0026] (1) Preparation of bis(4-fluorophenyl)phenylphosphine oxide (BFPPO)
[0027]
[0028] At room temperature, under a nitrogen atmosphere, slowly drop 66.0 ml (600.0 mmol, dissolved in 80.0 ml THF) of 4-fluorobromobenzene into a round bottom flask filled with 14.40 g (600.0 mmol) magnesium chips and 80.0 ml THF, dropwise After completion, heat to 60°C to react until the reaction of magnesium chips is complete, then cool the reaction system to 0°C, then slowly add 43.0 ml (300.0 mmol, dissolved in 80.0 ml THF) phenylphosphonic dichloride dropwise to the reaction solution, After the dropwise addition, the reaction system was warmed up to 80 o C for 9 h, then reacted at room temperature until the raw materials disappeared (TLC monitoring), then added 10% sulfuric acid solution to quench the reaction, adjusted the pH to 1, then extracted the aqueous phase with 300.0 ml ether for 3 times,...
Embodiment 2
[0043] Preparation of bis(4-chlorophenyl)(3'-diethoxyphosphonophenyl)phosphine oxide
[0044] (1) Preparation of bis(4-chlorophenyl)phenylphosphine oxide (BCPPO)
[0045]
[0046] At room temperature, under a nitrogen atmosphere, slowly drop 114.87g (600.0 mmol, dissolved in 80.0 ml THF) 4-chlorobromobenzene into a round bottom flask containing 14.40 g (600.0 mmol) magnesium chips and 80.0 ml THF, dropwise After completion, heat to 60 o C to react until the reaction of magnesium scraps is complete, then cool the reaction system to 0°C, slowly add 43.0 ml (300.0 mmol , dissolved in 80.0 ml THF) phenylphosphonic dichloride dropwise to the reaction solution, after the addition is complete The reaction system was heated to 80°C for 9 h, then reacted at room temperature until the raw materials disappeared (monitored by TLC), then quenched the reaction by adding 10% sulfuric acid solution, adjusted the pH to 1, and then extracted the aqueous phase with 300.0 ml diethyl ether thr...
Embodiment 3
[0060] Preparation of phosphonate polymer (I-1)
[0061]
[0062] (I-1)
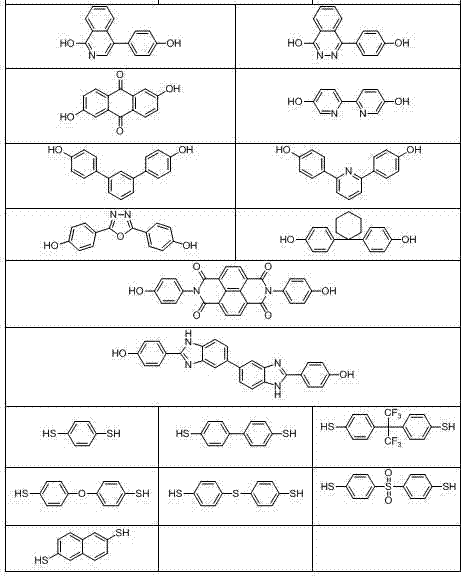
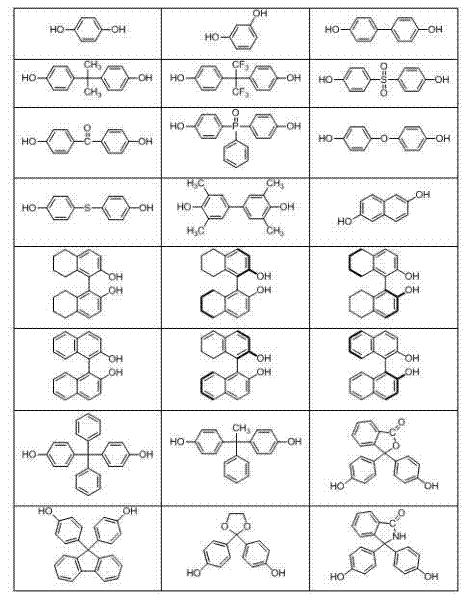
[0063] Under nitrogen atmosphere, bis(4-fluorophenyl)(3'-diethoxyphosphonophenyl)phosphine oxide (6.30 g, 14 mmol), 4,4'-dichlorodiphenylsulfone (1.72 g, 6 mmol), biphenol (3.72 g, 20 mmol), anhydrous potassium carbonate (3.18 g, 23 mmol), N,N-dimethylacetamide (DMAC) 56 mL, toluene 28 mL, mixed and heated Water was separated at 160°C for 4 hours, the toluene was distilled off, the temperature was raised to 180°C, and the reaction was carried out for 36 hours. Slowly pour the reaction solution into 400 mL of deionized water to obtain a white strip polymer, change the water, keep the temperature at 80°C, boil in water three times, each time for 6 hours, filter, dry, and vacuum dry at 100°C for 24 hours to obtain a white Fibrous polymer 10.31 g. Yield 96%, intrinsic viscosity 0.55 dL / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com