Curcumin salicylic acid monoester and synthesis method thereof and application curcumin salicylic acid monoester to anti-tumor and anti-inflammatory aspects
A technology of curcumin salicyloyl monoester and curcumin, which is applied in the field of pharmacy, can solve problems such as unstable properties, difficulty in reaching effective concentrations, and restrictions on the use range of curcumin, and achieve excellent anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 curcumin salicyloyl monoester
[0028] Under ice bath, 10 mmol (3.68 g) of curcumin was dissolved in 80 mL of anhydrous dichloromethane, and 7.5 mmol (1.035 g) of salicylic acid and catalyst DMAP (0.5 g) were added. Slowly add 10 ml of anhydrous dichloromethane dissolved with 2.22 g of DCC dropwise, continue to stir and react for 24 hours, reclaim the solvent to obtain a solid product, the product is dissolved in ethyl acetate, and purified by silica gel column chromatography, eluent toluene: acetic acid Ethyl ester=14:1, to obtain the pure product of yellow curcumin salicyloyl monoester.
[0029] Molecular formula C 28 h 24 o 8 , melting point 166°C, yellow crystal, 1 H NMR (D6-DMSO): 3.85(s, 6H, 2Ar-OCH 3 ), 6.15(s, 1H), 6.80(d, 2H, J=16Hz, -C H =CH-Ar), 6.83-7.19(m, 3H, Ar), 7.33(d, 1H, J=8Hz,), 7.38-7.61(m, 5H, Ar), 7.64(d, 2H, J=16Hz, -C H =CH-Ar) 8.01 (d, 1H, Ar), 9.69 (s, 1H, Ar-OH), 10.24 (s, 1H, Ar-OH), 13 C NMR(D6-DMSO):5...
Embodiment 2
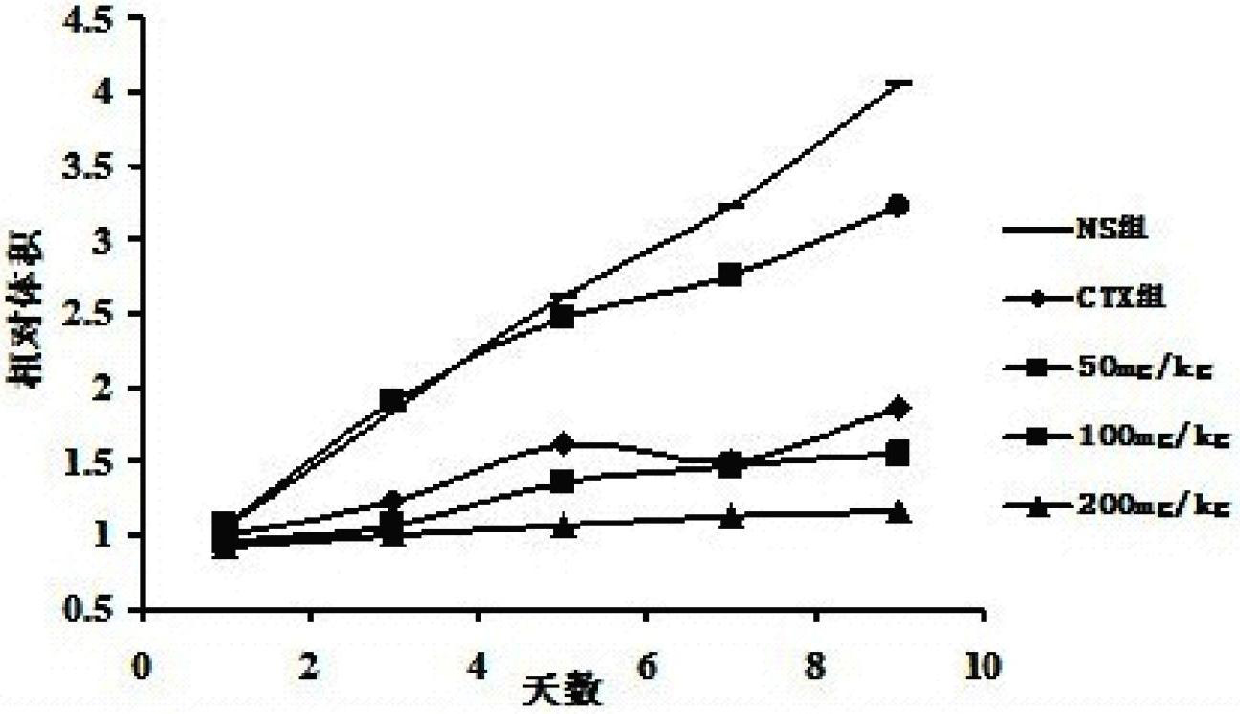
[0030] The verification of the in vitro antitumor activity of embodiment 2 curcumin salicyloyl monoester
[0031] 2.1 Material description
[0032] Tumor cell lines: human chronic myelogenous leukemia cell line K562; human acute leukemia cell line HL60; human multiple myeloma cell line U266; Burkitts lymphoma cell line CA46; human gastric cancer cell line SGC7901; human colon cancer cell line SW1116; Liver cancer cell line SMMC7721; human pancreatic cancer cell line PANC-1; human cervical cancer cell line Hela; human breast cancer cell line MCF-7; human nasopharyngeal carcinoma CNE2; human nasopharyngeal carcinoma CNE1; mouse melanoma cell line B16, The above cells were all obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0033] 2.2 Experimental method
[0034] Tumor cells were cultured in RPMI 1640 medium containing 10% calf serum, 100 IU / ml penicillin and 100 μg / ml streptomycin in an incubator at 37°C with 5% CO2 saturated humidity. Cells in the ...
Embodiment 3
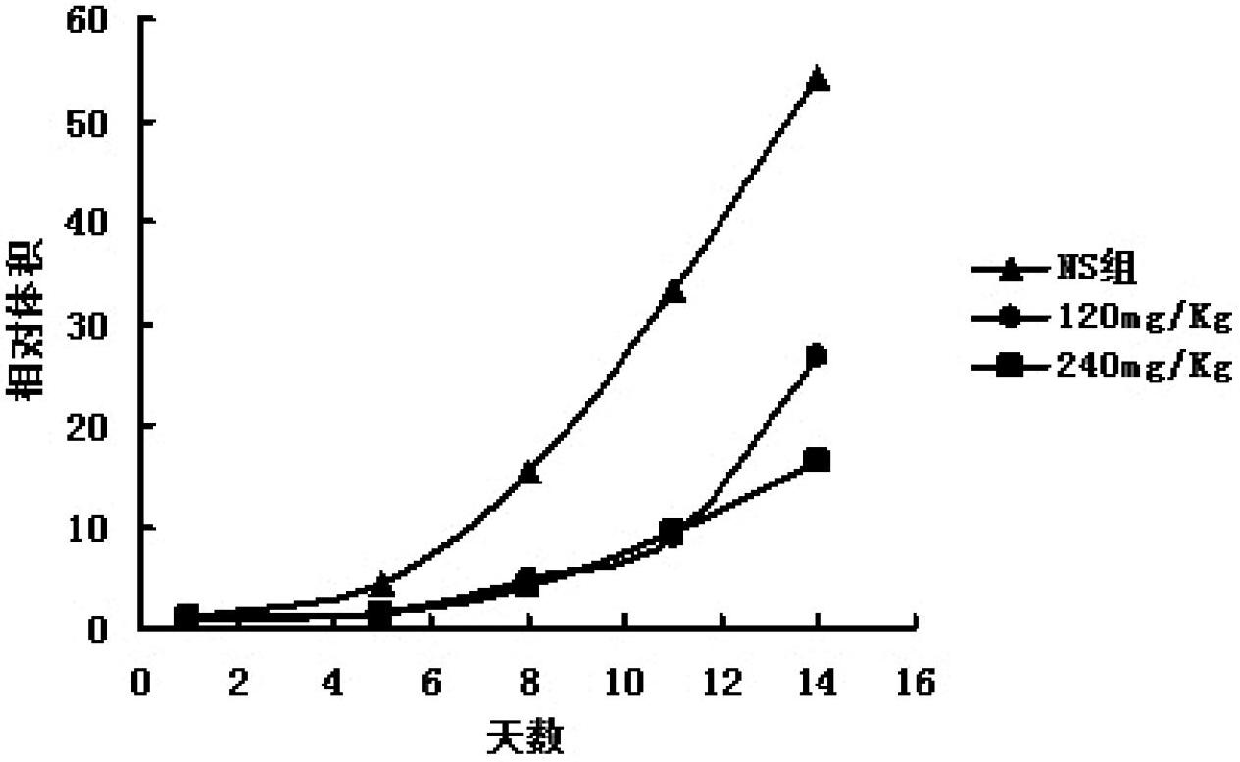
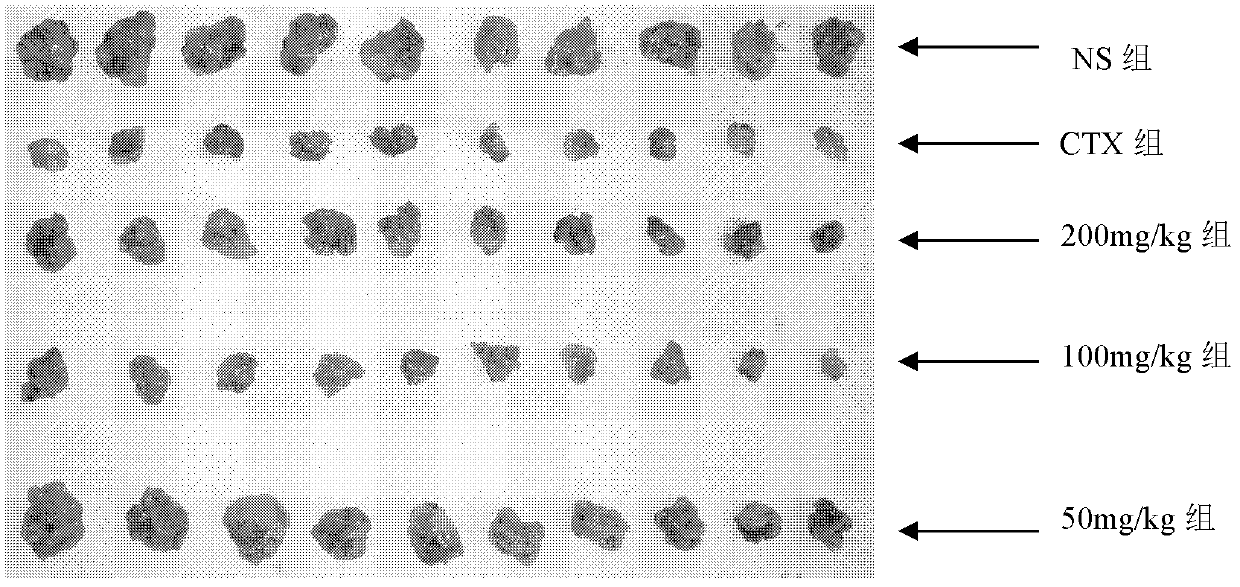
[0040] The effect of embodiment 3 curcumin salicyloyl monoester on mouse liver cancer H22 xenograft model
[0041] 3.1 Materials 8-12 weeks old Kunming healthy mice, weighing (20±2) g; the mice were provided by the Experimental Animal Center of Fujian Medical University. Mouse hepatoma cell line H22.
[0042] 3.2 Method
[0043] 3.2.1 Establishment of tumor-bearing mouse model
[0044] H22 tumor cells were passed on for more than two generations, and the number of cells was adjusted to 10 7 / ml, 0.2mL / only inoculated in the abdominal cavity of mice, inoculated 6 mice, waited for about two weeks, aseptically extracted ascites, added sterile normal saline to adjust the number of cells, and then inoculated (each inoculated cells were about 2×10 6 ).
[0045] 3.2.2 Grouping
[0046] Be divided into 5 groups altogether: the mouse after inoculating tumor strain 24h is divided into 5 groups at random: normal saline control group, curcumin salicyl monoester (following examples sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap