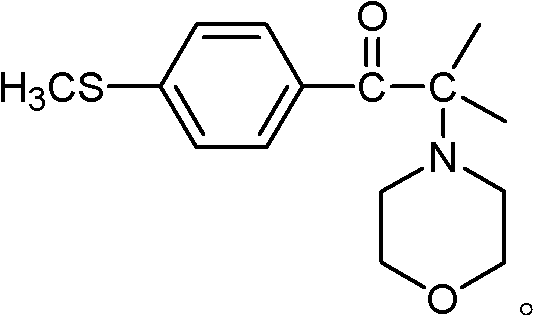
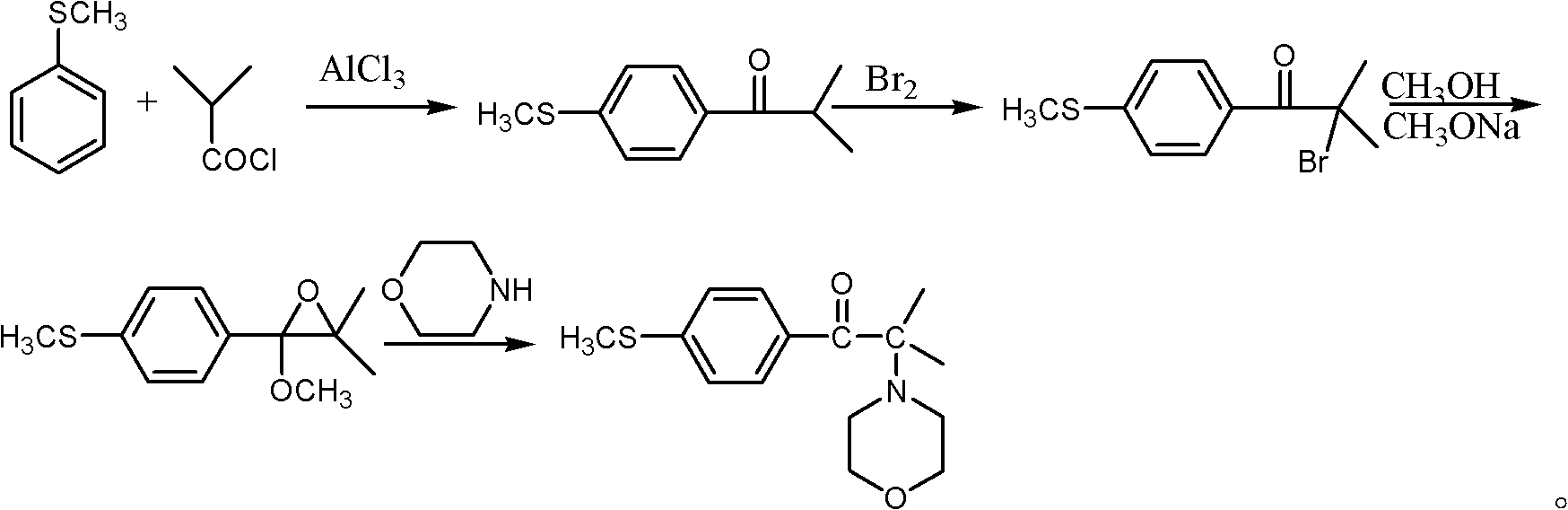
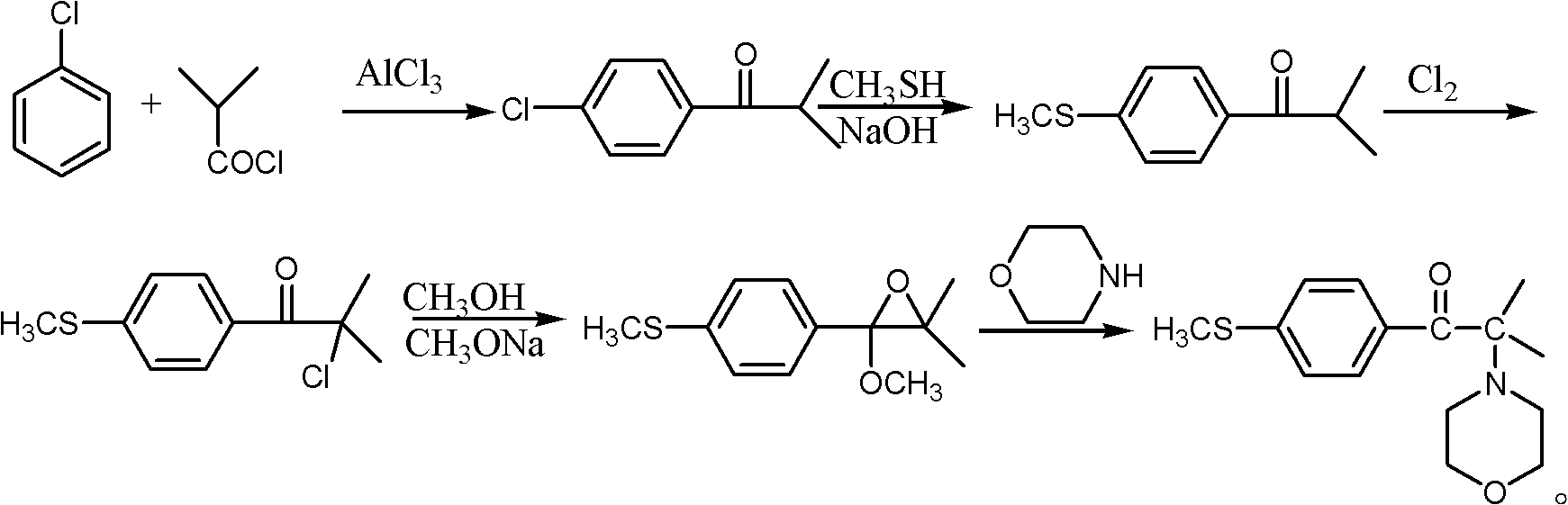
Synthetic method of 2-methyl-1-(4'-methylthiophenyl)-2-morpholinyl-1-acetone
The technology of a methylthiophenyl group and a synthesis method, which is applied in the field of organic chemical synthesis, can solve problems such as potential safety hazards in downstream use of photoinitiators, and achieve the effects of low cost of raw materials, simple and easily available raw materials, and strong competitive advantages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 11-(4'-chlorophenyl)-2-methyl-1,2-propylene oxide
[0050] In a 2000ml four-neck flask, first add 644g of p-chlorobenzyl chloride, then add 348g (5.6mol) of dimethyl sulfide, 300g of water, heat and reflux for 12 hours, the reaction temperature is 50-55°C, when the reaction liquid becomes homogeneous After the reaction is over, cool down to 38-40°C, add 232g (4mol) of acetone, then add dropwise 915g of 35% liquid caustic soda, slowly heat to 50°C after the dropwise addition, and take a sample to detect the acetone content after the reaction for 10 hours. Change, stop the reaction, lower the temperature to 20°C, let stand to separate the water layer, the organic layer is firstly distilled at normal pressure to recover acetone and dimethyl sulfide, and then distilled under reduced pressure to collect the fraction at 90-100°C / -0.09MPa to obtain 1- (4'-Chlorophenyl)-2-methyl-1,2-propylene oxide, 657 g of fractions were collected.
[0051] Prod...
Embodiment 2
[0053] a) In a 2000ml flask, dissolve 365g (2mol) of 1-(4'-chlorophenyl)-2-methyl-1,2-propylene oxide in 200ml methanol, add 522g (5mol) morpholine, 20g Catalyst, heated to 80°C and refluxed for 8 hours, sampled and analyzed, GC detected that 1-(4'-chlorophenyl)-2-methyl-1,2-propylene oxide disappeared, first distilled methanol at normal pressure, and then started Distill under reduced pressure, recover the remaining methanol and excess morpholine, cool the concentrate to 50°C, add 200ml of toluene and stir to dissolve, filter out the catalyst, then add 360g of hydrochloric acid aqueous solution with a concentration of 20% by mass, and adjust the pH to 7. Leave to stand for stratification, transfer the water layer to another flask to participate in the next reaction, and recycle the toluene in the upper organic layer.
[0054] The preparation method of the catalyst is as follows: Weigh 1g of phosphotungstic acid (PW), 100g of Y-type molecular sieve and 10g of activated alumina...
Embodiment 3
[0060] a) In a 2000ml flask, 1-(4'-chlorophenyl)-2-methyl-1,2-propylene oxide 365g (2mol) was dissolved in 200ml methanol, and 565g (6.5mol) morpholine was added, 20g catalyst, heated up to 80°C and refluxed for 8 hours, sampled and analyzed, GC detected that 1-(4'-chlorophenyl)-2-methyl-1,2-epoxypropane disappeared, first distilled methanol at normal pressure, and then Start vacuum distillation, reclaim the remaining methanol and excess morpholine, cool to 50°C, add 200ml of toluene and stir to dissolve, filter out the catalyst, then add 360g of hydrochloric acid aqueous solution with a mass percentage concentration of 20%, adjust the pH=7, statically Set the layers, transfer the water layer to another flask to participate in the next reaction, and recycle the toluene in the upper organic layer.
[0061] The preparation method of the catalyst is as follows: Weigh 1g of phosphotungstic acid (PW), 100g of Y-type molecular sieve and 25g of activated alumina respectively, mix the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com