Indometacin and albuterol suppository, preparation method, detection method and application thereof
A technology of indomethacin albuterol suppository and indomethacin, which is applied in the directions of suppository delivery, anti-inflammatory agent, measuring device, etc., can solve problems such as sedimentation and stratification, influence on curative effect and safe medication, patient discomfort, etc. safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment is used to illustrate the preparation method of the indomethacin salbutamol suppository provided by the present invention.
[0034] Prepare a suppository with a specification of 1.7 g / capsule, prepare 1000 capsules and add 70 g of indomethacin, and the total weight of the matrix is 1630 g. According to the test, to meet the requirements of preparation melting time limit, hardness, and delamination resistance, the matrix composition is formulated as follows: (the total weight of the matrix=the weight of 1000 suppositories-medicine powder weight, i.e. 1630g)
[0035]
[0036] 1) Mix fatty acid glycerides, polyoxyethylene sorbitol beeswax derivatives, glyceryl behenate, and methylparaben according to the recipe and melt to 75°C; 2) Heat polyoxyethylene (25) stearate to 70°C, add indomethacin and albuterol to melt for later use; 3) Mix the result of step 1) and step 2), homogeneously emulsify at 2000-6000rpm for 5-20min, cool the interlayer cooling wate...
Embodiment 2
[0038] This embodiment is used to illustrate the preparation method of the indomethacin salbutamol suppository provided by the present invention.
[0039] Prepare a suppository with a specification of 1.7 g / capsule, prepare 1000 capsules and add 70 g of indomethacin, and the total weight of the base is 1630 g. According to the test, to meet the requirements of preparation melting time limit, hardness, and delamination resistance, the matrix composition is formulated as follows: (the total weight of the matrix=the weight of 1000 suppositories-medicine powder weight, i.e. 1630g)
[0040]
[0041]
[0042] 1) Mix fatty acid glycerides, polyglyceryl isostearate, glyceryl behenate, and ethyl paraben according to the prescription and melt to 75°C; 2) Heat polyoxyethylene (40) stearate to 70°C ℃, add indomethacin and salbutamol for later use; 3) mix the result of step 1) and step 2), homogeneously emulsify at 2000-6000rpm for 5-20min, cool the interlayer cooling water to 50 ℃, ...
Embodiment 3
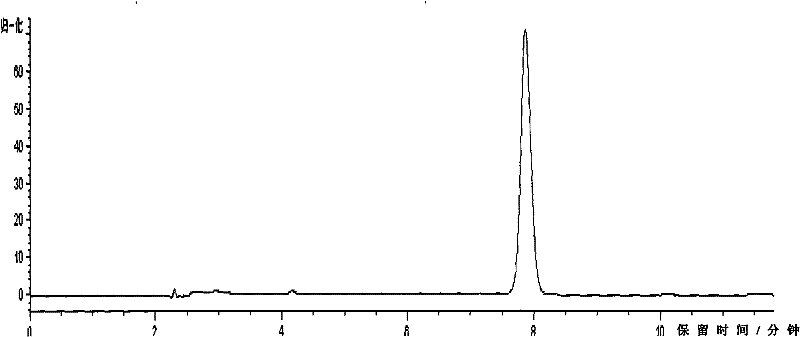
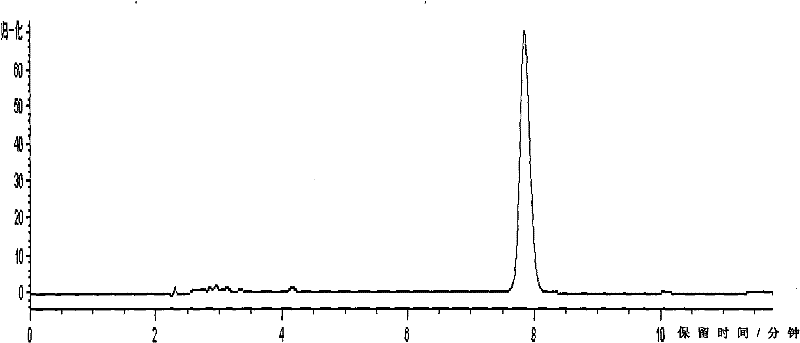
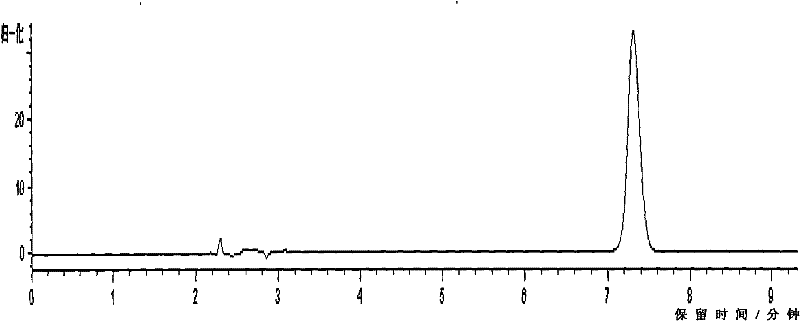
[0044] This embodiment is used to illustrate the detection of the indomethacin salbutamol suppository provided by the present invention.
[0045] In the suppository prepared by the prescription process of the present invention, the main drug is uniformly dispersed, and the auxiliary materials do not affect the content determination.
[0046] 1. The quality control detection method of albuterol is as follows:
[0047]1) Liquid chromatography column: use octadecylsilane bonded silica gel as filler (4.6mm×150mm, 5μm), use phosphate buffer (take 9.77g sodium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, use Phosphoric acid to adjust the pH value to 3.10±0.05)-methanol (85:15) as the mobile phase, the detection wavelength is 276nm, the number of theoretical plates should not be less than 2000 based on the calculation of the salbutamol sulfate peak, the flow rate is 1.0mL / min, and the detection wavelength is 276nm , the injection volume is 100 μl;
[0048] 2) D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com