Catalyst used in preparation of low-carbon olefins by using synthesis gas and preparation method and application of catalyst
A low-carbon olefin and catalyst technology, which is applied to the catalyst and preparation field for the direct synthesis of low-carbon olefins from synthesis gas (hydrogen and carbon monoxide), can solve problems such as long process routes, and achieve high product selectivity, low production cost, and single-pass conversion. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
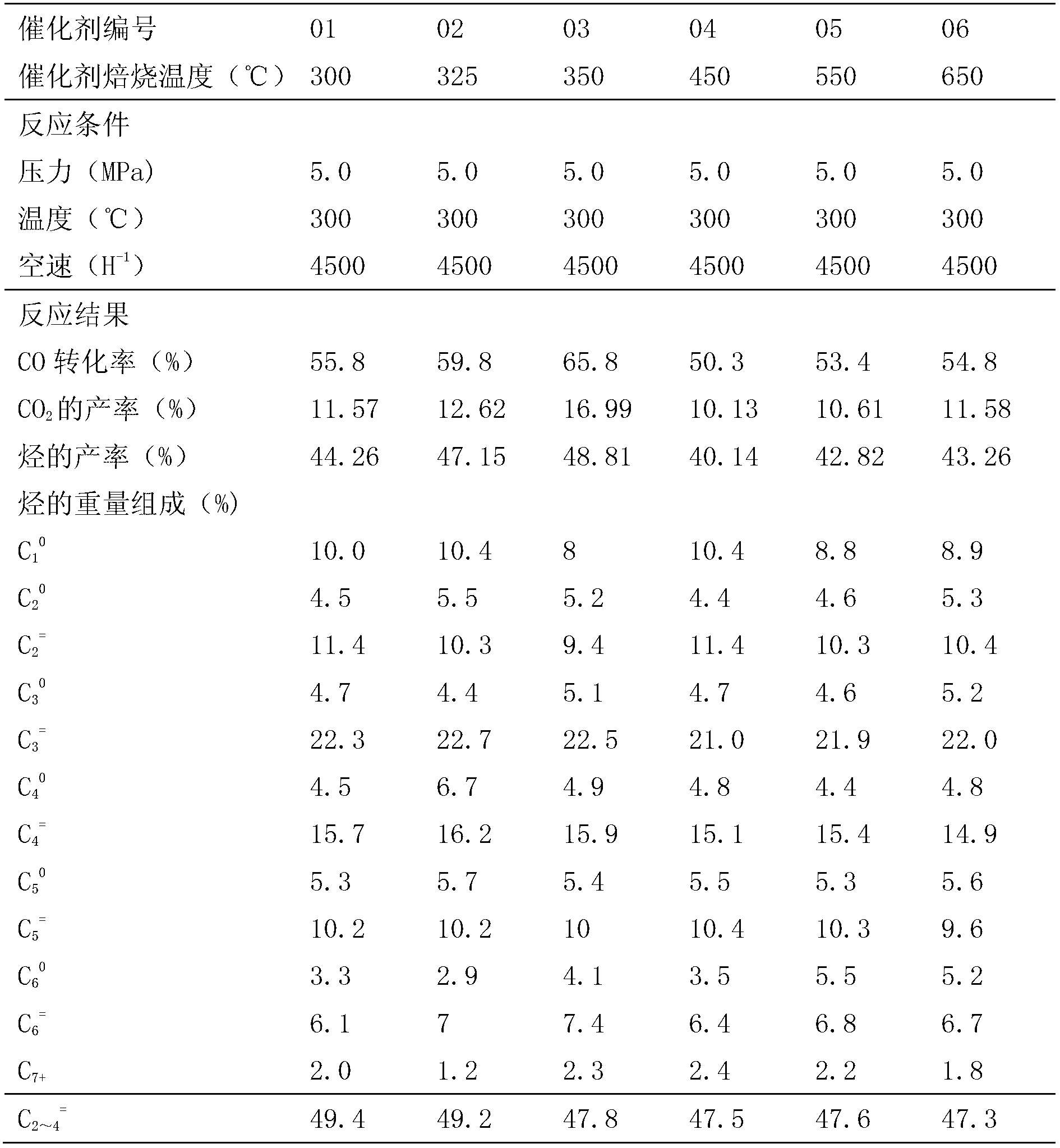
[0025] Preparation of No. 01-06 main active component iron oxide and zinc oxide composite oxide catalyst
[0026] Weigh 12.1 grams of ferric nitrate and 9.45 grams of zinc nitrate and add 100 ml of deionized water to prepare a mixed solution; weigh 26.5 grams of sodium carbonate and add 100 ml of deionized water to prepare a 20% solution by weight. The two solutions are fed in parallel at 70°C, and the pH is controlled to be about 9±0.2. After the feeding is completed, continue to stir for 30 minutes, and then age at 80°C for 2 hours. After the material is washed with water, it is dried in an oven at 120°C for 12 hours, then calcined at 300-650°C for 4 hours, and then cooled to room temperature to obtain a calcined iron oxide and zinc oxide composite oxide catalyst. Six catalysts obtained at different calcination temperatures are numbered 01-06. The mass composition of the synthesized catalysts is: ZnO 43.9% and FeO 56.1%, wherein the molar ratio of Fe and Zn atoms is 1:1.
...
Embodiment 2
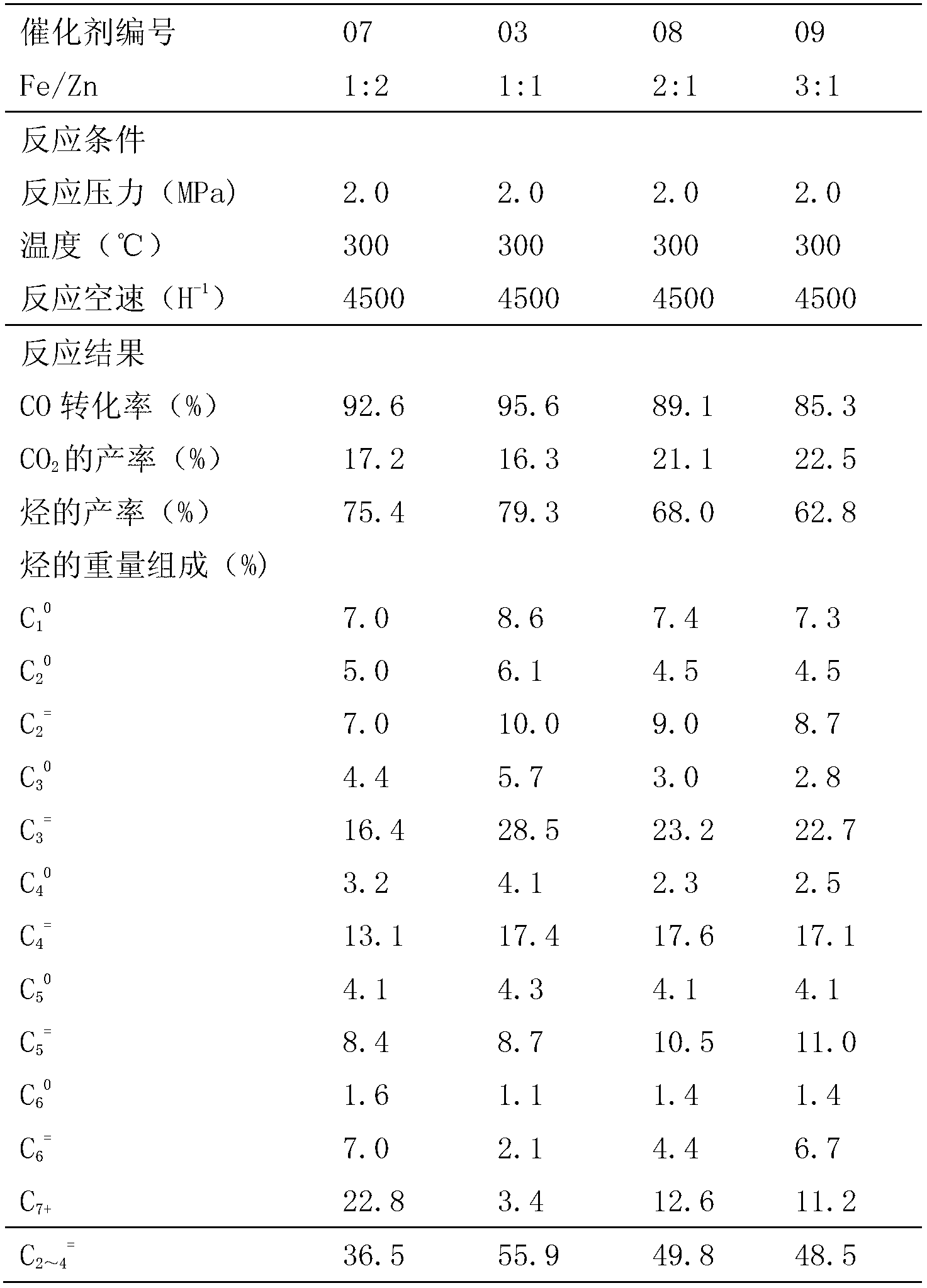
[0032] Preparation of No. 03, No. 07-09 main active component iron oxide and zinc oxide composite oxide catalyst
[0033] The aqueous solution of different weights of ferric nitrate and zinc nitrate was used to prepare the catalyst in the same manner as in Example 1, and the obtained precipitate was dried in an oven at 120°C for 12 hours, then calcined at 350°C for 4 hours, and then cooled to room temperature to obtain Iron oxide and zinc oxide composite oxide catalyst, the molar ratio of Fe and Zn atoms is 1:2, 2:1 and 3:1, and the catalyst number is 07-09. The above-mentioned catalyst and 03 catalyst were evaluated in a fixed-bed reactor, and the results obtained by online analysis after two hours of reaction are shown in Table 2.
[0034] Table 2 Catalyst reaction results with different Fe / Zn ratios
[0035]
[0036] From the above data, it can be seen that in the range of Fe / Zn=1:2~3:1, the CO conversion rate can exceed 85%. When Fe / Zn=1:2, the selectivity of light ole...
Embodiment 3
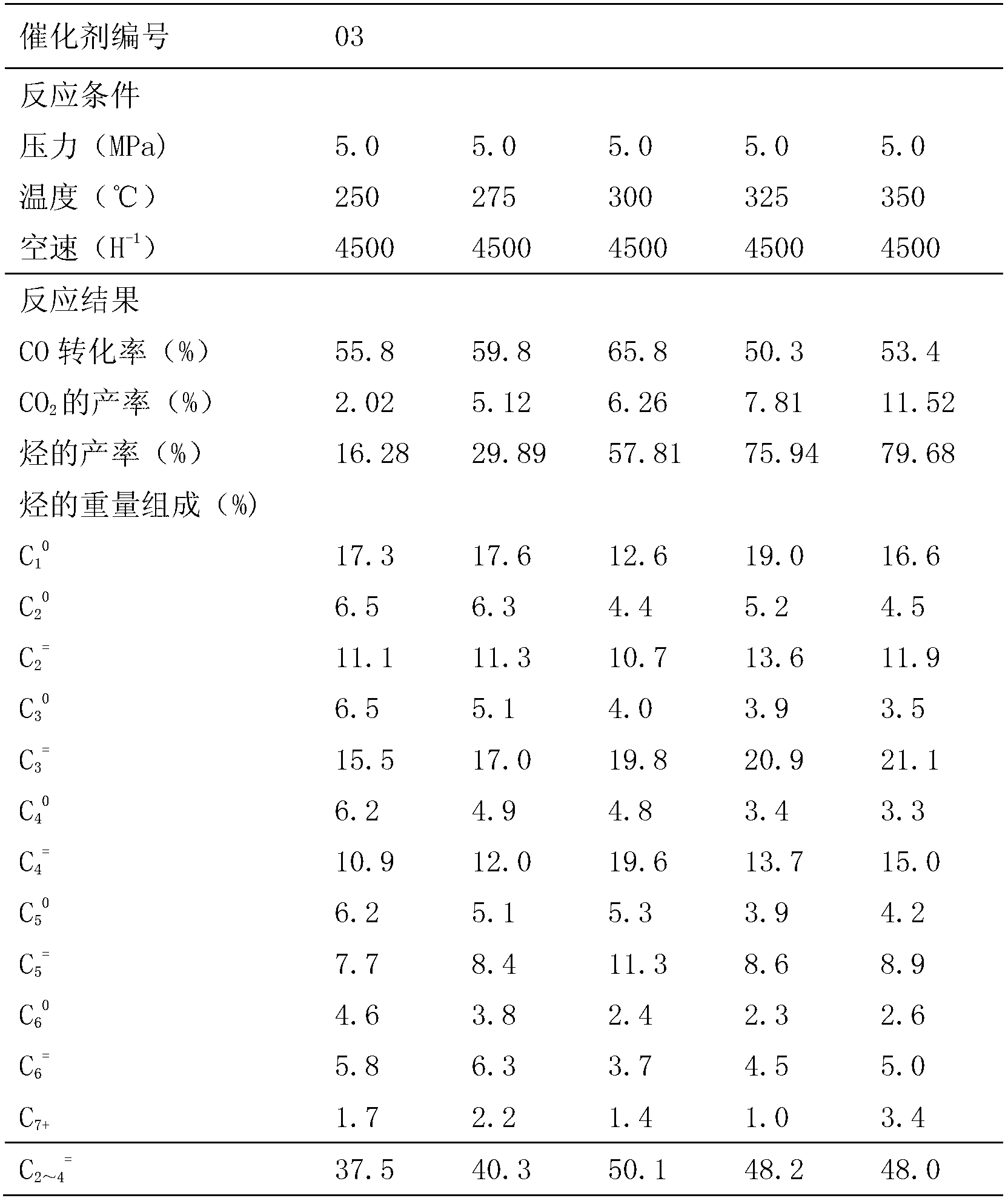
[0038] The performance evaluation results of the No. 03 main active component iron oxide and zinc oxide composite oxide catalyst under different conditions of temperature, pressure and reaction volume space velocity are shown in Tables 3-5.
[0039] The influence of table 3 different reaction temperature on catalyst performance
[0040]
[0041] The data in Table 3 show that when the reaction temperature is lower than 300 ° C, the selectivity of light olefins is low, and the temperature increases, and the CO 2 The yield increases, and when the reaction temperature is 300°C, catalyst No. 03 has better selectivity of light olefins and higher reactivity.
[0042] Evaluation results of catalysts under different pressures in table 4
[0043]
[0044] As the reaction pressure increases, the CO conversion rate increases slightly, indicating that the increase in reaction pressure is beneficial to the improvement of catalyst activity, but the increase in reaction pressure reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com