Aramagnetic epoxy group mesoporous molecular sieve for immobilized biological enzymes, and preparation method thereof
An epoxy-based mesoporous and mesoporous molecular sieve technology is applied in biochemical equipment and methods, immobilized enzymes, molecular sieve compounds, etc., to achieve the effects of facilitating diffusion, improving separation efficiency, and improving operational stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
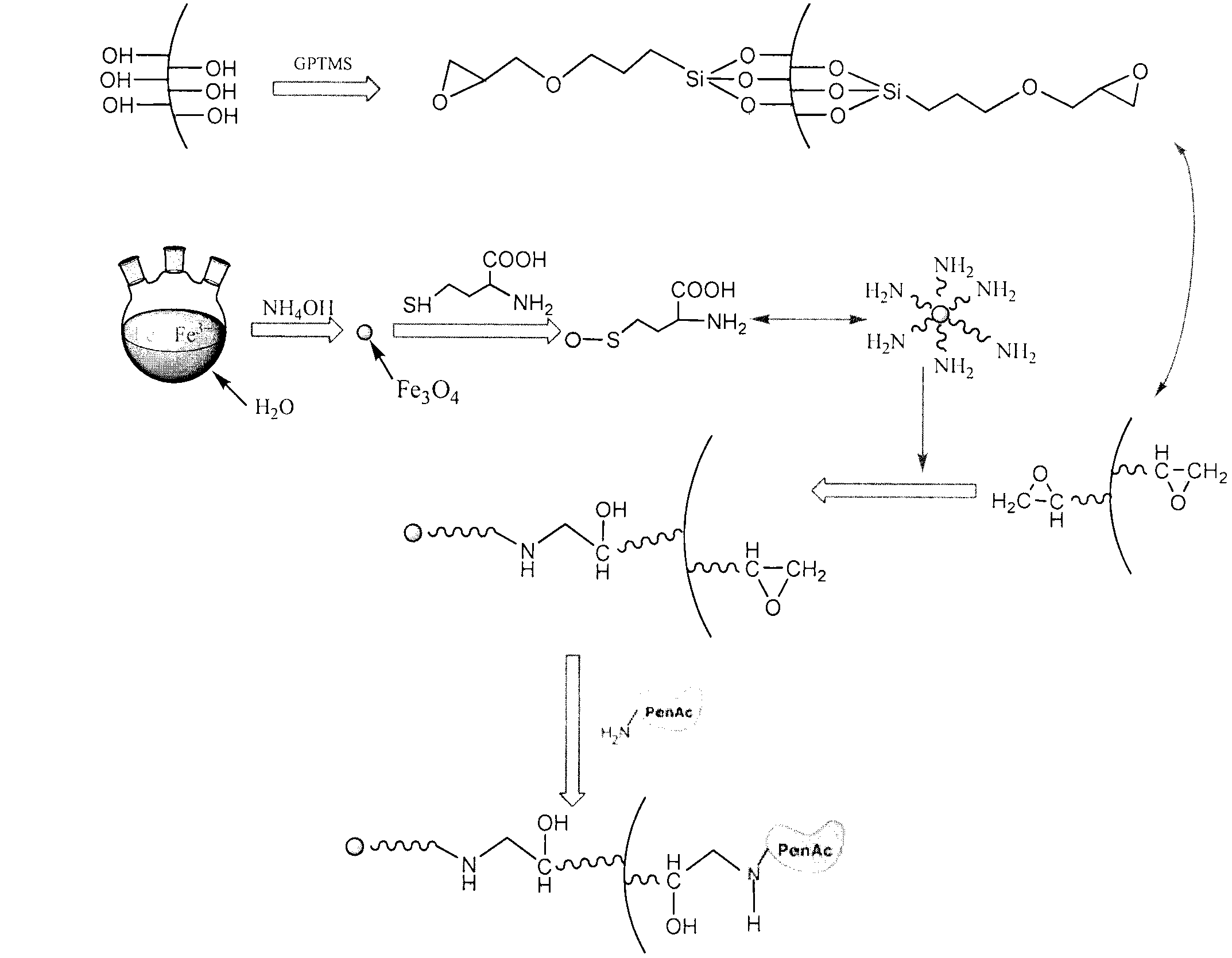
[0036] 0.365g FeCl 3 ·6H 2 O and 0.208g FeSO 4 ·7H 2 O(Fe 3+ / Fe 2+ The molar ratio is 1.8:1) dissolved in 10mL of water and heated to 80°C, adjusted to a pH of about 11 with concentrated ammonia water, aged at this temperature for 2 hours, cooled to room temperature and washed with water until neutral to obtain 0.15g of Fe 3 o 4 Then disperse the nanoparticles in 20mL water, adjust the pH value of the solution to 4.0-5.0 with dilute hydrochloric acid, then add 5mL of L-cysteine solution with a concentration of 5g / L, react with ultrasound for 30 minutes, and finally remove the aqueous solution under an external magnetic field , to obtain L-cysteine surface-modified Fe 3 o 4 Nanoparticles.
[0037] The epoxy-based mesoporous molecular sieve prepared in 0.60g comparative example and the Fe of the L-cysteine surface modification of 0.15g 3 o 4 The nanoparticles were respectively dispersed in 25mL of water, and then mixed uniformly and reacted at room temperature f...
Embodiment 2
[0039] Change the weight of epoxy-based mesoporous molecular sieve into 0.70g and L-cysteine surface-modified Fe in embodiment 1 3 o 4 The weight of the nanoparticles was changed to 0.30g, and other preparation processes were the same as in Example 1. The prepared paramagnetic epoxy-based mesoporous molecular sieve was used for the immobilization of penicillin acylase, and the immobilized enzyme activity obtained was 8514U / g, After 10 cycles of use, the immobilized enzyme retained 92.8% of its initial activity.
Embodiment 3
[0041] Change the weight of the epoxy-based mesoporous molecular sieve into the Fe of 0.45g and L-cysteine surface modification in embodiment 1 3 o 4 The weight of the nanoparticles was changed to 0.30g, and other preparation processes were the same as in Example 1. The prepared paramagnetic epoxy-based mesoporous molecular sieve was used for the immobilization of penicillin acylase, and the activity of the immobilized enzyme obtained was 8489U / g, After 10 cycles of use, the immobilized enzyme retained 89.8% of its initial activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com