Dimethylamine borane preparation method
A technology of dimethylamine borane and dimethylamine hydrochloride, applied in the field of compound preparation, can solve the problems of difficult control of reaction temperature, long reaction time, unsuitable for industrial production, etc., and achieves great application value, simple method and yield improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
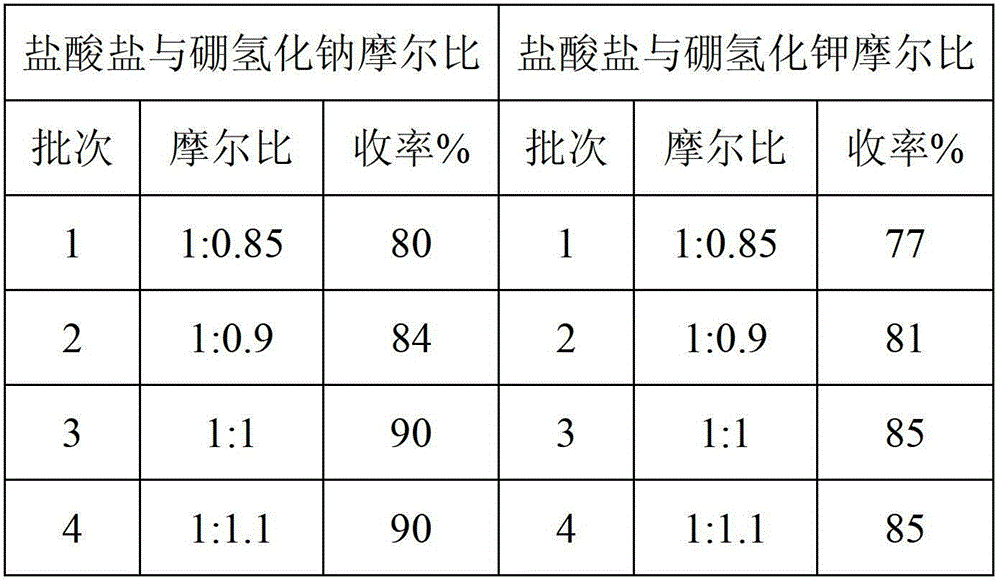
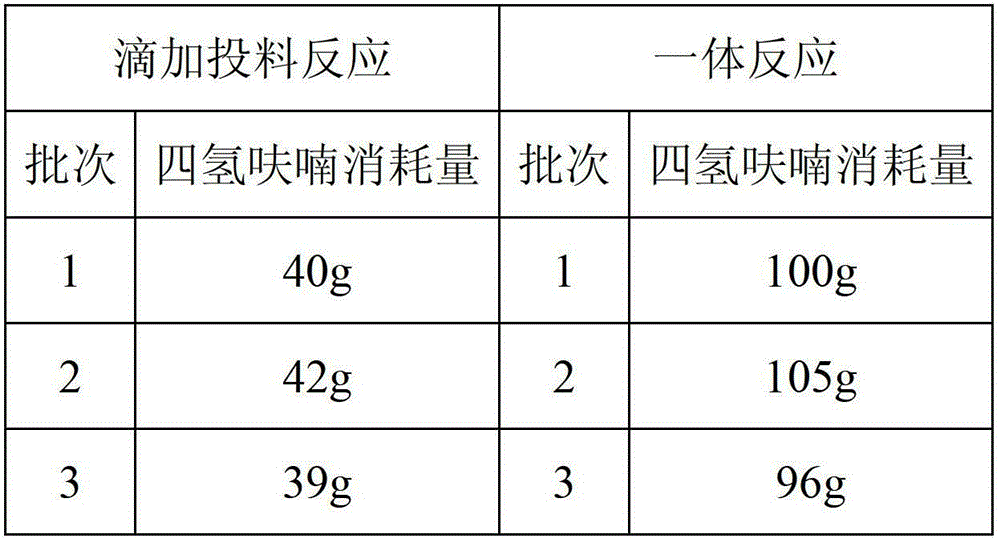
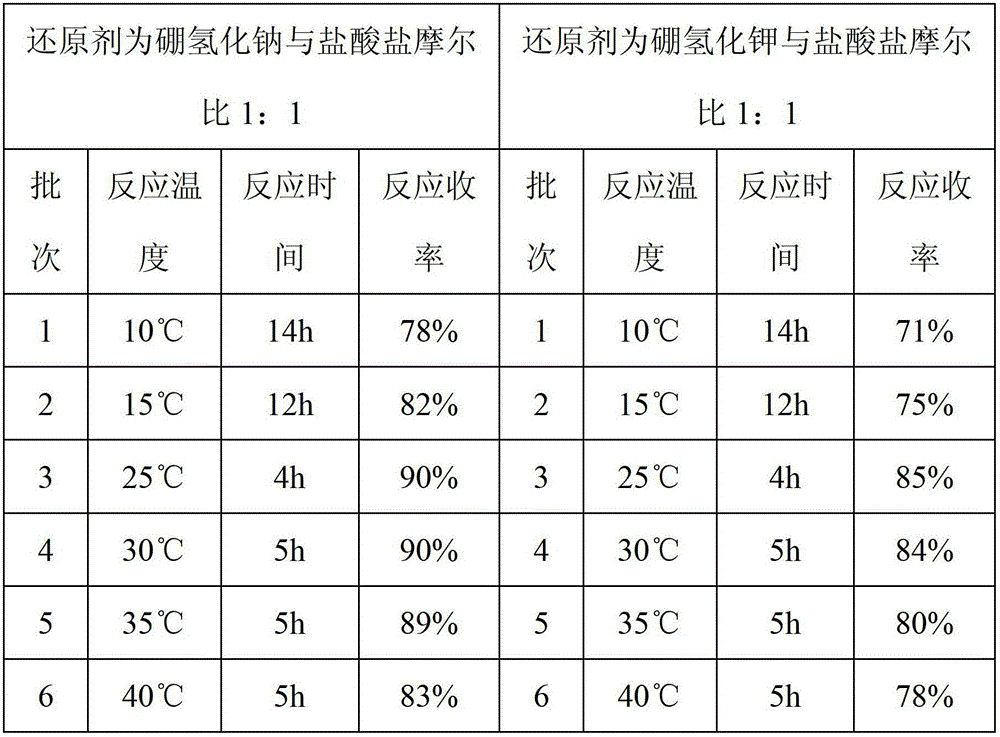
[0022] Add 81.55g (1mol) of dimethylamine hydrochloride and 150g of tetrahydrofuran into a 500ml reaction flask, and stir to mix. 37.83 g (1 mol) of sodium borohydride and 150 g of tetrahydrofuran were added to another reactor and stirred. Add dimethylamine hydrochloride / tetrahydrofuran dropwise to sodium borohydride / tetrahydrofuran, keep the internal temperature at 25-30°C, and stir for 4 hours. Then add 150g (25°C) saturated sodium chloride / 3% sodium hydroxide solution to the reaction system, stir at 25°C for 0.5 hour, stand still for 0.5 hour, and remove the lower layer solution. Add 10 g of sodium hydroxide to the upper layer solution, stir for 0.5 hour, stand still for 0.5 hour, and remove the lower alkaline aqueous layer. The supernatant was filtered and then distilled under normal pressure (25-85°C) and then under reduced pressure (0.09MPa, 25-85°C) to recover 250g of tetrahydrofuran. In the concentrated solution obtained after distillation, add 20g of 30% sodium hydr...
Embodiment 2
[0024] Add 81.55g (1mol) of dimethylamine hydrochloride and 150g of tetrahydrofuran into a 500ml reaction flask, and stir to mix. 53.83 g (1 mol) of potassium borohydride and 150 g of tetrahydrofuran were added to another reactor and stirred. Add dimethylamine hydrochloride / tetrahydrofuran dropwise to sodium borohydride / tetrahydrofuran, keep the internal temperature at 25-30°C, and stir for 4 hours. Then add 150g (25°C) saturated potassium chloride / 3% sodium hydroxide solution to the reaction system, stir at 25°C for 1 hour, stand still for 1 hour, and remove the lower layer solution. Add 10 g of sodium hydroxide to the upper layer solution, stir for 1 hour, stand still for 1 hour, and remove the lower alkali water layer. The supernatant was filtered and then distilled under normal pressure (25-85°C) and then under reduced pressure (0.09MPa, 25-85°C) to recover 250g of tetrahydrofuran. Add 20g of 30% sodium hydroxide solution to the concentrated solution obtained after disti...
Embodiment 3
[0026] With above-mentioned example 1, after adding sodium hydroxide solution to the concentrated solution after example 2 distillation, after directly adding the water of 9 times of solid weight amount, can be made into 10% dimethylamine borane aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com