Method for preparing tetrahydro-2-ethyl anthraquinone from working solution for production of hydrogen peroxide by anthraquinone process
A technology of ethyl anthraquinone and working fluid, applied in quinone separation/purification, organic chemistry, etc., can solve the problems of high price, complicated operation, high cost, etc., and achieve the effect of low cost, easy to obtain raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
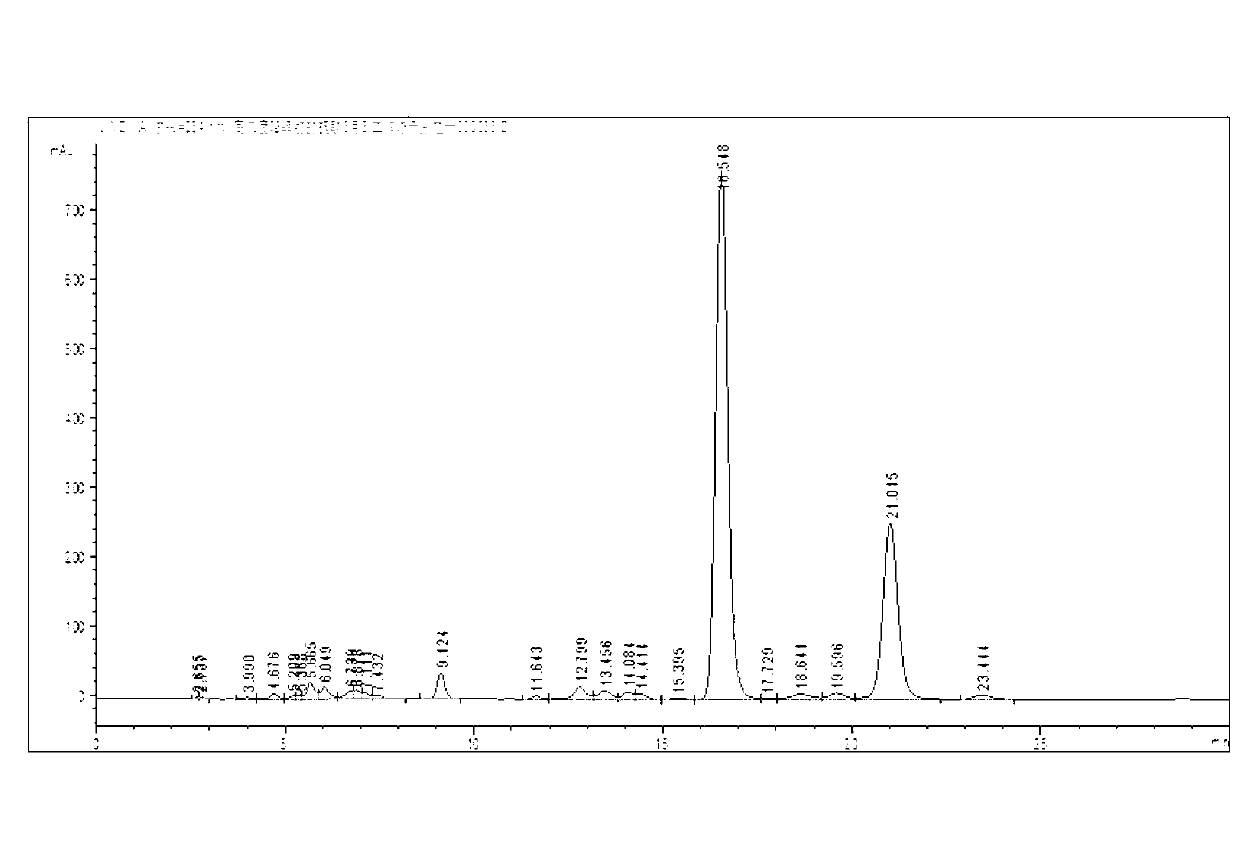
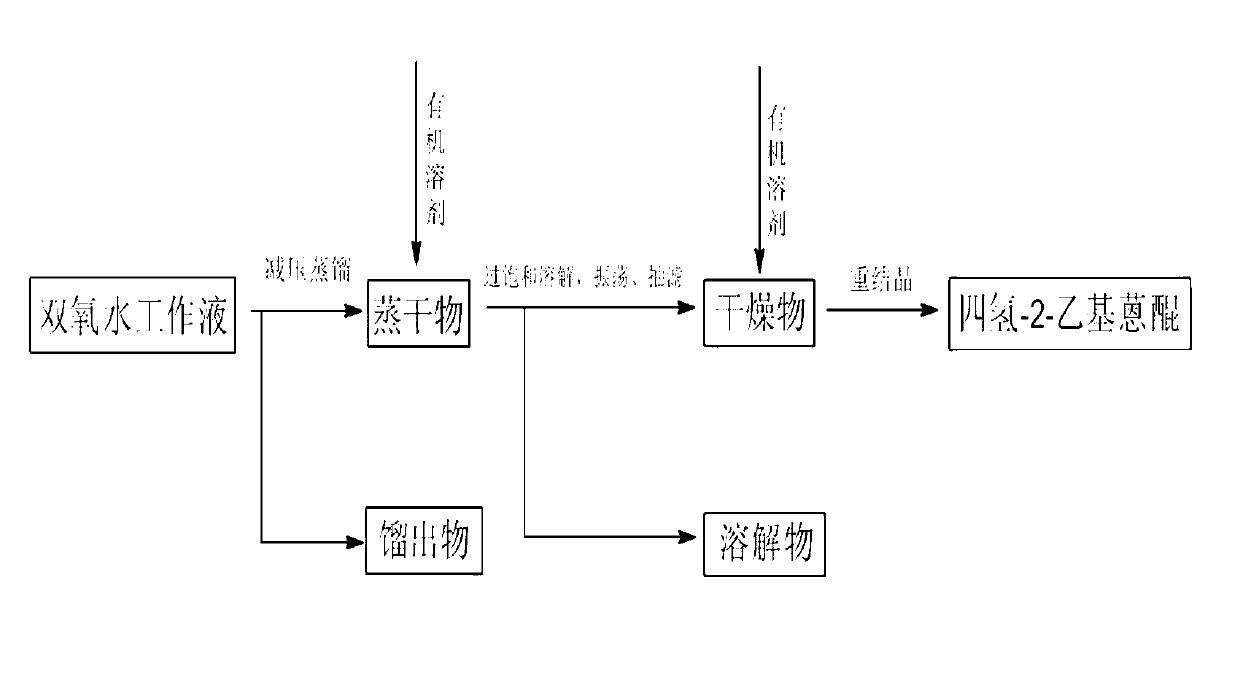
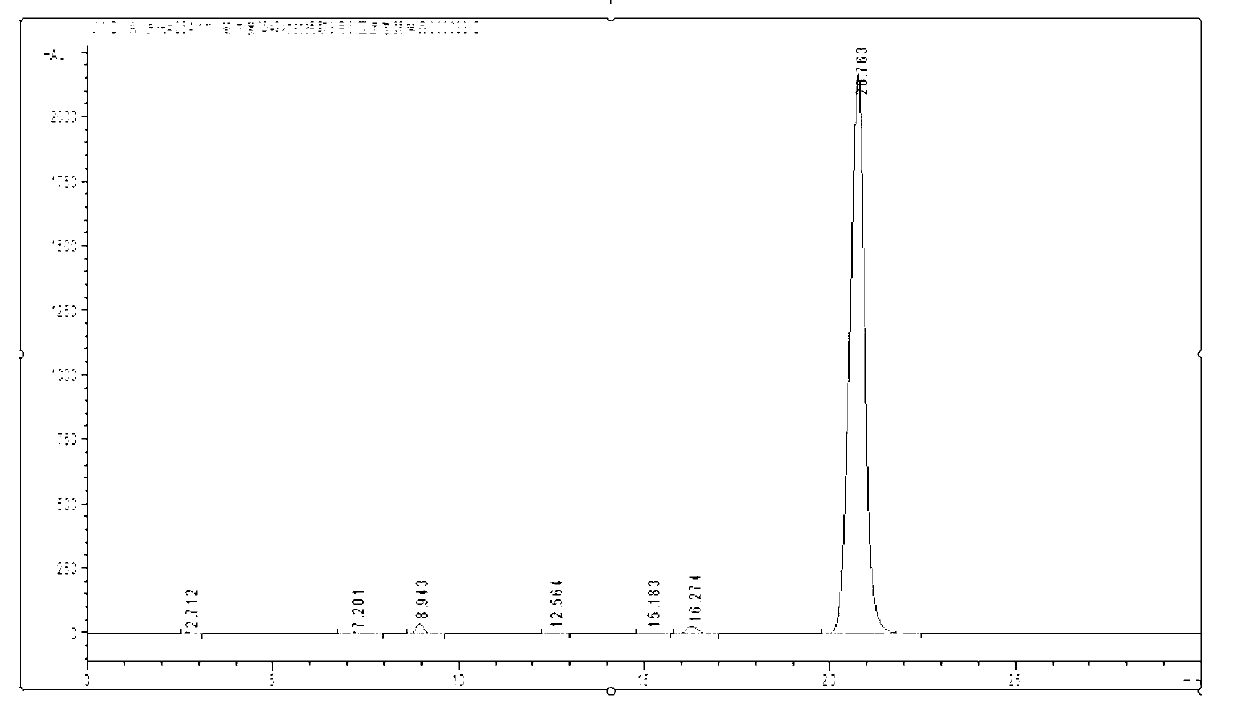
[0032] Take 400mL hydrogen peroxide working solution, adjust the oil bath to 160°C on a rotary evaporator, adjust the vacuum pump to 0.2MPa, distill under reduced pressure for 6 hours, and cool to obtain the evaporated product of the working solution. Take 30g of the evaporated matter and add 15mL of absolute ethanol, stir well at 8°C for 0.5h until the insoluble matter in the evaporated matter is completely powdery, filter with suction, and dry the insoluble matter at 60°C to obtain a dry matter, which is Dissolve in N-methylpyrrolidone, the quality of N-methylpyrrolidone is 10 times of the dry matter, stir to dissolve the dry matter completely, add distilled water drop by drop to the solution, stir while adding, until there is a small amount of yellow precipitate Precipitate, filter with suction, wash the yellow precipitate with a large amount of distilled water, and dry to obtain tetrahydro-2-ethylanthraquinone with a purity of 98%.
Embodiment 2
[0034]Take 400mL hydrogen peroxide working solution, adjust the oil bath to 160°C on a rotary evaporator, adjust the vacuum pump to 0.2MPa, distill under reduced pressure for 6 hours, and cool to obtain the evaporated product of the working solution. Take 30g of the evaporated matter and add 15mL of absolute ethanol, stir well at 8°C for 0.5h until the insoluble matter in the evaporated matter is completely powdery, filter with suction, and dry the insoluble matter at 60°C to obtain a dry matter, which is Dissolve in N-methylpyrrolidone, the mass of N-methylpyrrolidone is 10 times that of the dry matter, stir to dissolve the dry matter completely, add distilled water drop by drop to the solution, stir while adding, until there is a large amount of yellow precipitate Precipitation, suction filtration, so that the obtained recrystallization is about half of the amount of dry matter put into it, recrystallize the first recrystallization again, the quality of the second recrystalli...
Embodiment 3
[0036] Take 400mL hydrogen peroxide working solution, adjust the oil bath to 160°C on a rotary evaporator, adjust the vacuum pump to 0.2MPa, distill under reduced pressure for 6 hours, and cool to obtain the evaporated product of the working solution. Take 30g of the evaporated matter and add 15mL of n-hexane, fully stir at 8°C for 0.5h until the insoluble matter in the evaporated matter is completely powdery, filter with suction, dry the undissolved matter at 60°C to obtain a dried matter, dissolve the dried matter Add the acetone to acetone, the quality of acetone is 10 times that of the dry matter, stir to dissolve the dry matter completely, add distilled water drop by drop to the solution, stir while adding, until a large amount of yellow precipitates are precipitated, filter with suction, and remove the yellow precipitate with Wash with copious amounts of distilled water and dry. After three recrystallizations, tetrahydro-2-ethylanthraquinone with a purity of 98% can be o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com