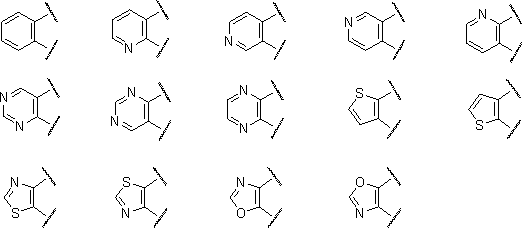
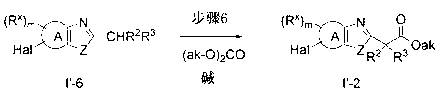Oxadiazole derivative having endothelial lipase inhibitory activity
A compound and solvate technology, applied in the field of oxadiazole derivatives with vascular endosebase inhibitory activity, can solve the problems of record of HDLc elevation without EL inhibitory effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
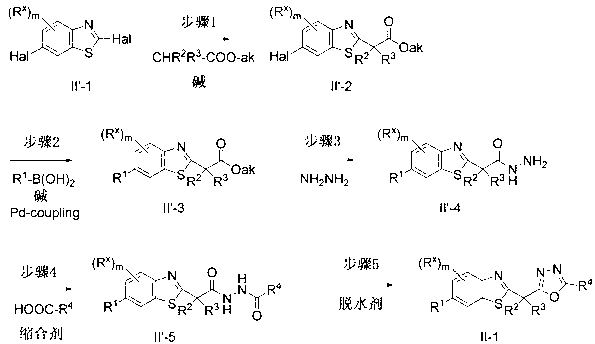
[0545] [chemical 40]
[0546]
[0547] To a solution of 2 M NaHMDS in THF (111 mL, 211 mmol) in 375 mL of anhydrous toluene was added ethyl acetate (11.30 mL, 116 mmol) dropwise over 10 min at -60 °C under nitrogen flow. Then it was stirred at -60°C for 1 hour, and then a solution of 6-bromo-2-chlorobenzothiazole 1 (25 g, 101 mmol) in 125 mL of anhydrous toluene was added dropwise. After completion of the dropwise addition, the mixture was stirred at 0° C. for 2 hours.
[0548] 1 M hydrochloric acid and ethyl acetate were added to the reaction solution for extraction, and the organic layer was washed with saturated brine and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the residue was washed with a mixed solvent of hexane and diisopropyl ether to obtain Compound 2 (27.1 g, 90%) as a yellow solid.
[0549] Compound 2: 1H-NMR (CDCl3) δ: 1.31 (t, J = 7.2, 3.0Hz, 3H), 4.15 (s, 2H), 4.26 (q, J = 7.2 Hz, 2H), 7.57 (dd, J = 8.7, 1.8Hz,...
Embodiment 2
[0551] [chem 41]
[0552]
[0553] At room temperature, tetrakis(triphenylphosphine)palladium(0) (5.39 g, 4.66 mmol), phenyl Boric acid (9.75 g, 80 mmol), K 3 PO 4 (35.4 g, 167 mmol), heated to reflux for 6 hours. The reaction was returned to room temperature, 1 M hydrochloric acid and ethyl acetate were added for extraction, and the organic layer was washed with saturated brine and dried over sodium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by column chromatography to obtain Compound 3 (16.1 g, 81%) as a yellow solid.
[0554] Compound 3: 1 H-NMR (CDCl3) δ: 1.31(t, J = 6.9 Hz, 3H), 4.19 (s, 2H), 4.27 (q, J =7.2 Hz, 2H), 7.38 (t, J = 7.5Hz, 1H) , 7.48 (t, J = 7.2Hz, 2H), 7.63-7.73 (m, 3H), 8.06 (m, 2H).
[0555] Hydrazine hydrate (7.99 mL, 165 mmol) was added to a solution of compound 3 (9.8 g, 33 mmol) in 100 mL of anhydrous N-methylpyrrolidone, and stirred at 95°C for 7 hours. After returning the reaction...
Embodiment 3
[0566] [chem 42]
[0567]
[0568] To a solution of compound 4 (600 mg, 2.2 mmol) in 45 ml of anhydrous dimethylformamide was added successively 2-(tert-butoxycarbonylamino)acetic acid (464 mg, 2.65 mmol) at room temperature under a stream of nitrogen , WSCD HCl (609 mg, 3.18 mmol) and HOBt (86 mg, 0.635 mmol), stirred for 4 hours. 1 M hydrochloric acid and ethyl acetate were added to the reaction solution for extraction, and the organic layer was washed with saturated brine and dried over sodium sulfate. Ethyl acetate and hexane were added to the residue, and the precipitated insoluble matter was collected by filtration to obtain compound 6 (640 mg, 69%).
[0569] Compound 6: 1H-NMR (DMSO-d6) δ: 1.42 (s, 9H), 3.59 (d, J = 6.4Hz, 2H), 4.11 (s, 2H), 7.01 (br-s, 1H), 7.39 ( t, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.73-7.81 (m, 3H), 8.01 (d, J = 8.8 Hz, 1H), 8.38(s, 1H), 10.19 (br s, 1H).
[0570] Burgess reagent (730 mg, 3.1 mmol) was added to a solution of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com