Construction of brucellosis A19 molecular marker vaccine strain and determination of virulence and immunogenicity
A molecular marker vaccine, brucellosis technology, applied in the determination/examination of microorganisms, bacteria, recombinant DNA technology, etc. The effect of weak strength, improved vaccine function, and short market cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Construction of recombinant suicide plasmid
[0036] Materials and Methods
[0037] Strains, plasmids, vectors
[0038] The Brucella A19 strain was purchased from the China Veterinary Drug Administration, and the suicide plasmid pBK-CMV-sacB was preserved by the Veterinary Research Department of the Xinjiang Academy of Animal Sciences; the cloned pGEM-T vector was purchased from Promega, a commercial reagent.
[0039] Reagent
[0040] Restriction enzymes were purchased from Fermentas; plasmid DNA extraction kit and DNA gel recovery kit were purchased from Promega; T4DNA ligase, DNA Marker, and DH5α were purchased from Beijing Zhuangmeng Biological Company; sequence determination was provided by Shanghai Invitrogen Trading Co., Ltd. Ltd. completed.
[0041] Primers were designed according to the GenBankAF141604 sequence, as shown in Table 1:
[0042] Table 1 Primer Sequence
[0043]
[0044] PCR amplification
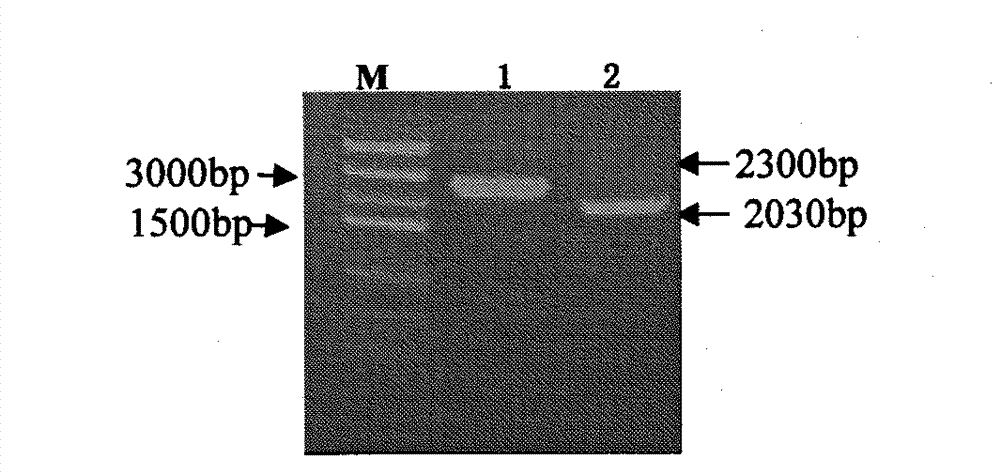
[0045] Amplification of Homology Arm Fragment Upstrea...
Embodiment 2
[0068] Described is the determination of the virulence and immunogenicity of the brucellosis A19 molecular marker vaccine strain.
[0069] Characteristics of A19 molecular marker strain:
[0070] Experimental animals were 4-6 week old BALB / C female mice purchased from Xinjiang Experimental Animal Research Center, license number: SCXK (new) 2003-0002.
[0071] Safety test: Dilute A19-ΔVirB12 viable bacteria with normal saline to contain 1 billion viable bacteria per 1ml, subcutaneously inject 5 BABL / C mice with a body weight of 18-20g, each 0.25ml, and all live healthy within 10 days.
[0072] Genetic Stability:
[0073] Passage stability: Streak culture of the frozen A19-ΔVirB12 molecular marker strain on a tryptone soybean broth medium plate at 37°C with 5% CO 2 Cultured in the incubator for 72 hours, the grown colony was the first-class seed, and the first-class seed was continuously passed on the medium for 50 generations, and the colonies of the passaged bacteria were pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com