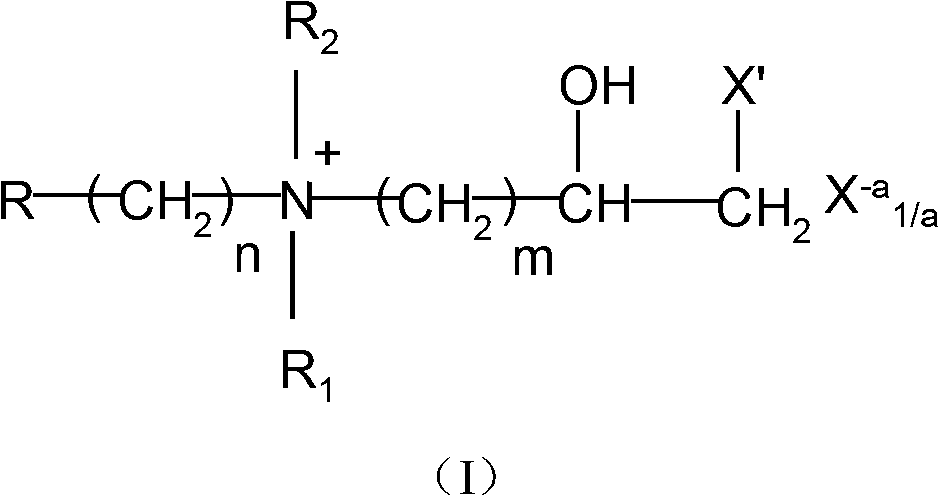
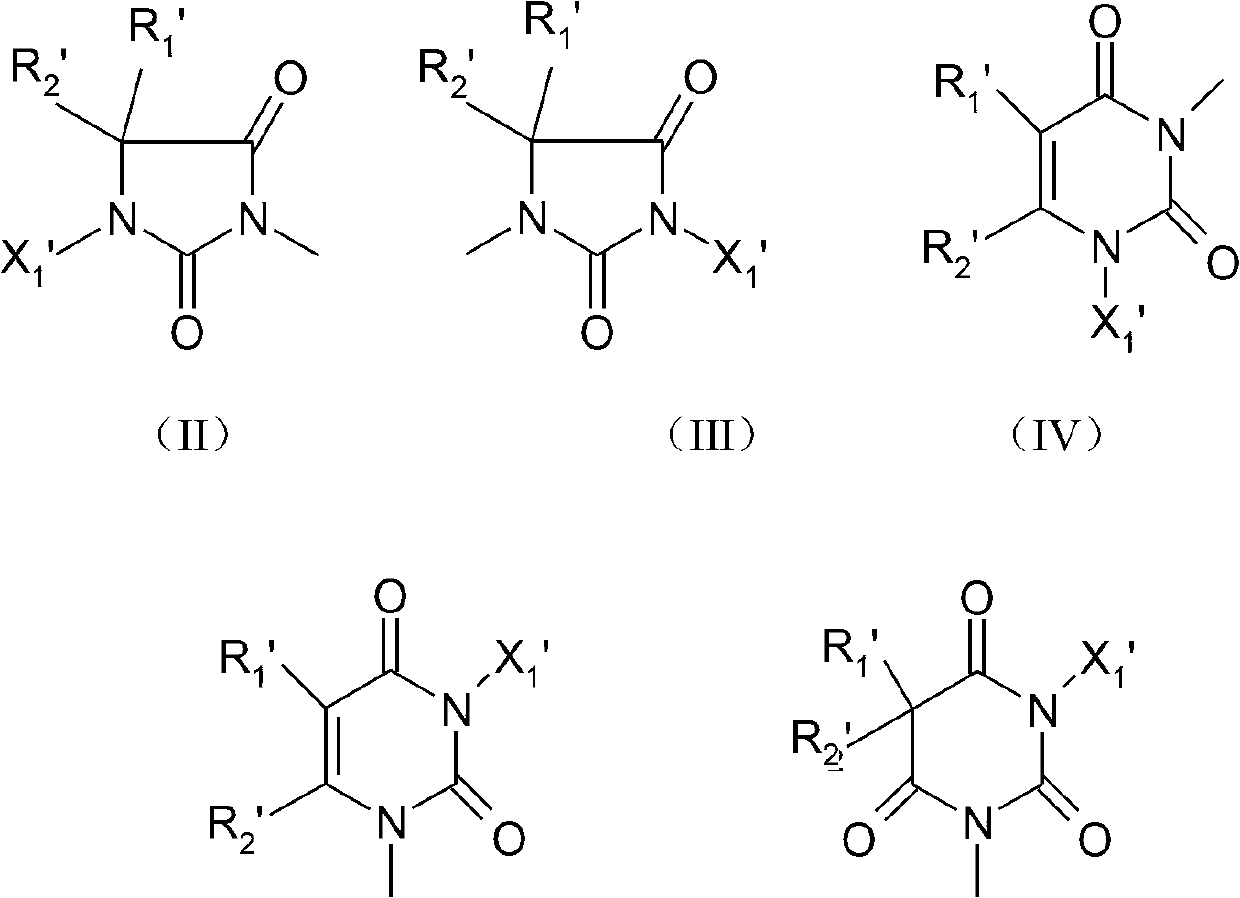
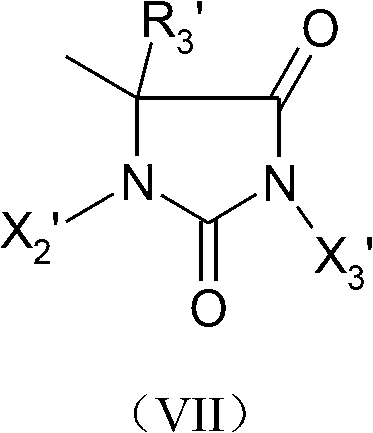
Hydroxy halogen amine compound containing quaternary ammonium salt functional group and preparation method and application thereof
A technology of quaternary ammonium salt function and hydroxyhalamine, which is applied in the synthesis and application of halamine fungicides, and the synthesis and application of quaternary ammonium salted hydroxyhalamine compounds, which can solve the problem of decreased surface hydrophilicity and insufficient antibacterial efficacy. Long-lasting, affecting material softness and wrinkle resistance, etc., to achieve the effect of broad-spectrum bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Synthesis of 1-dimethylaminomethyl-5,5-dimethylhydantoin
[0084]
[0085] Add 15.80 g (0.10 mol) of 1-hydroxymethyl-5,5-dimethylhydantoin to a 500 ml round bottom flask filled with 200 ml of isopropanol, stir to dissolve, then add 16.39 g (0.12 mol) A 33% aqueous solution of dimethylamine was stirred and reacted at 30°C for 4 hours, and the solvent was distilled off from the reaction solution under reduced pressure to obtain 17.99 g of crude product, which was obtained by titration with hydrochloric acid to obtain 1-dimethylaminomethyl-5,5- The content of dimethyl hydantoin is 99.86%, and the yield of reaction can be calculated accordingly to be 97.11%. Take 2.00 grams of crude product, add an appropriate amount of anhydrous acetone until it is completely dissolved, and then pass through dry hydrogen chloride gas, there is a large amount of solids, namely 1-dimethylaminomethyl-5,5-dimethylhydantoin The hydrochloride was precipitated, centrifuged, and drie...
Embodiment 2
[0086] Example 2 Synthesis of (2,3-dihydroxypropyl)-(5,5-dimethylhydantoin-1)methyl-dimethylammonium chloride
[0087]
[0088] Add 18.50 g (0.10 mol) of 1-dimethylaminomethyl-5,5-dimethylhydantoin to a 500-ml round-bottomed flask filled with 200 ml of water, heat to dissolve at 55°C and add 9.25 grams (0.10mol) of epichlorohydrin, stirred for 4 hours, measured by silver nitrate titration (2,3-dihydroxypropyl)-(5,5-dimethylhydantoin-1)methyl- The yield of dimethylammonium chloride is about 83.47%. The solvent was distilled off under reduced pressure to obtain 28.61 g of a crude product. Dissolve the crude product in absolute ethanol, add anhydrous acetone to crystallize and purify the product, and centrifuge to separate (2,3-dihydroxypropyl)-(5,5-dimethylhydantoin-1)methyl-dimethyl ammonium chloride solid, and then dried in a vacuum oven. The molecular ion peak M of the quaternary ammonium salt positive ion was obtained by mass spectrometry + : 260.2. IR:3261cm -1 Lef...
Embodiment 3
[0089] The synthesis of embodiment 3 (3-chloro-2-hydroxypropyl)-(5,5-dimethylhydantoin-1) methyl-dimethyl ammonium chloride
[0090]
[0091] Add 18.50 g (0.10 mol) of 1-dimethylaminomethyl-5,5-dimethylhydantoin to a 500-ml round-bottomed flask filled with 200 ml of 0.50 mol / L hydrochloric acid solution and wait for it to dissolve Finally, 9.25 g (0.10 mol) of epichlorohydrin was added, stirred and reacted at room temperature for 6 hours, and the reaction ended when the solution was slightly alkaline. The solvent was distilled off from the reaction solution under reduced pressure to obtain 30.81 g of a crude product. Dissolve the crude product in absolute ethanol, add anhydrous acetone to crystallize and purify the product, and centrifuge to obtain (3-chloro-2-hydroxypropyl)-(5,5-dimethylhydantoin-1)methyl-dimethyl ammonium chloride solid, and then dried in a vacuum oven. Elemental analysis test value: C 11 h 21 0 3 N 3 Cl 2 : N,13.26; C,41.52; H,6.91 (theoretical ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com