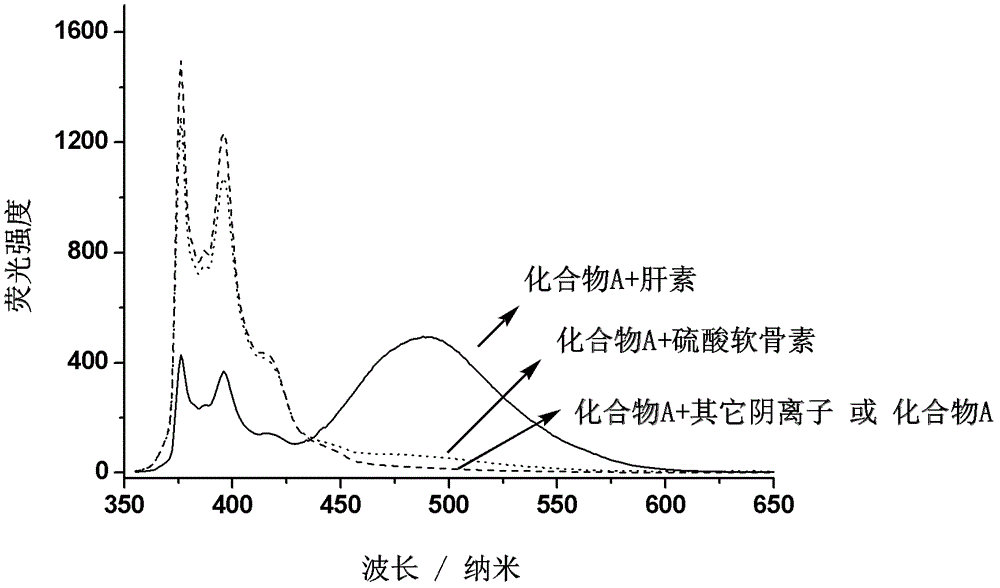
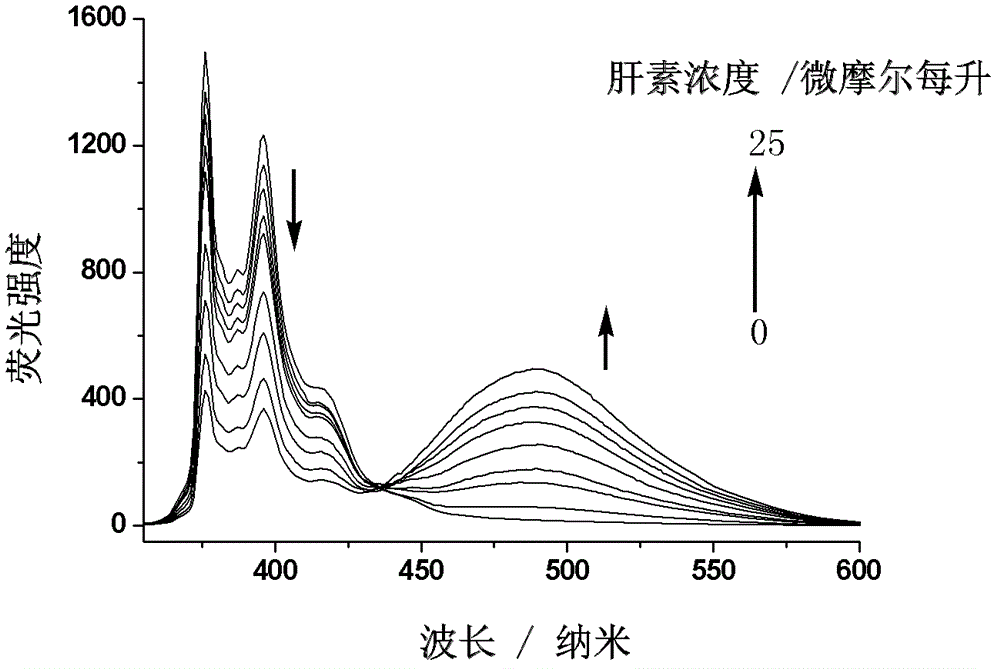
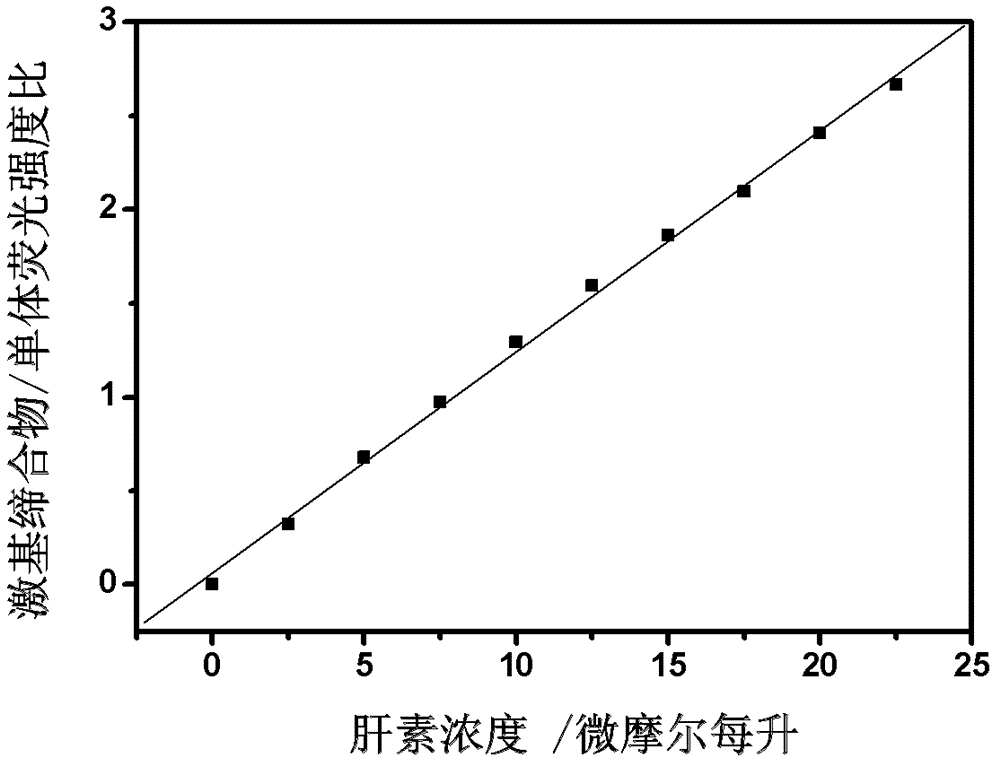
Fluorescent probe for quantitatively detecting heparin, and synthesis method and application thereof
A technology of fluorescent probes and synthesis methods, which is applied in the fields of biological analysis and chemical analysis, and can solve problems such as low sensitivity, large errors, and short fluorescence emission wavelengths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preparation of monoquaternary ammonium salt diamine includes the following steps:
[0069] Dissolve tetramethylethylenediamine in methanol, then add 0.8 times the molar amount of tetramethylethylenediamine to tetradecyl bromide, and react at 60°C for 10 hours. After concentrating the reaction solution, add water and use organic Solvent extraction and separation and separation by column chromatography, dichloromethane / methanol mixed solvent (volume ratio 8 / 1) as eluent, white solid compound: N, N, N', N'-tetramethyl-N -Tetradecyl ethylene diamine a.
[0070] 1 H NMR (400MHz, DMSO): δ 0.85 (t, 3H), 1.25 (m, 22H), 1.73 (m, 2H), 2.27 (s, 6H), 2.80 (t, 2H), 3.24 (t, 2H) ), 3.30(s, 6H), 3.34(t, 2H);
[0071] 13 C NMR(100MHz, CDCl 3 ): δ 14.2, 20.7, 22.7, 28.1, 29.3, 29.6, 31.8, 45.6, 51.7, 52.2, 60.8, 64.4. ESI-MS m / z=313.39M + , Calcd for C 20 H 45 N 2 + 313.36.
Embodiment 2
[0073] The preparation of monoquaternary ammonium salt diamine includes the following steps:
[0074] Dissolve tetramethylpropanediamine in N,N-dimethylformamide, add 0.8 times the molar amount of tetramethylpropanediamine to tetradecyl bromide, react at 50°C for 10 hours, and concentrate the reaction solution Then, water was added, the layers were extracted with organic solvent, and separated by column chromatography. The mixed solvent of dichloromethane / methanol (volume ratio 9 / 1) was used as eluent to obtain a white solid compound: N, N, N', N '-Tetramethyl-N-tetradecylpropanediamine b.
[0075] 1 H NMR (400MHz, DMSO): δ 0.85 (t, 3H), 1.26 (m, 22H), 1.73 (m, 2H), 1.83 (m, 2H), 2.27 (s, 6H), 2.36 (t, 2H) ), 3.24(t, 4H), 3.30(s, 6H);
[0076] 13 C NMR(100MHz, CDCl 3 ): δ14.1, 20.1, 20.7, 22.7, 28.1, 29.3, 29.6, 31.8, 45.9, 52.5, 57.6, 62.4, 64.7. ESI-MS m / z=327.39M + , Calcd for C 21 H 47 N 2 + 327.37.
Embodiment 3
[0078] The preparation of monoquaternary ammonium salt diamine includes the following steps:
[0079] Dissolve tetramethylhexamethylenediamine in a mixed solvent of dichloromethane and anhydrous methanol (the volume ratio of dichloromethane and anhydrous methanol is 6:1), add 0.8 times the molar amount of tetramethylhexamethylenediamine Methyl bromotetradecyl carboxylate was reacted at 30°C for 6 hours. After the reaction solution was concentrated, it was obtained by silica gel column chromatography using a dichloromethane / methanol mixed solvent (volume ratio 15 / 1) as the eluent White solid c.
[0080] 1 H NMR (400MHz, DMSO): δ, 1.26 (m, 6H), 1.29 (m, 10H), 1.30 (m, 2H), 1.68 (m, 2H), 1.73 (m, 2H), 1.83 (m, 2H) ), 2.25(t, 2H), 2.27(t, 6H), 2.36(t, 2H), 3.24(t, 4H), 3.30(s, 6H), 3.58(s, 3H).
[0081] 13 C NMR(100MHz, CDCl 3 ): δ20.1, 20.7, 25.0, 28.1, 29.0, 29.3, 29.6, 33.6, 45.4, 51.9, 52.5, 57.6, 62.4, 64.7, 173.1. ESI-MS m / z=371.36M + , Calcd for C 22 H 47 N 2 O 2 + 371.36.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com