Hydrotalcite tablet and its preparation method
A technology of magnesium carbonate tablets and magnesium carbonate, applied in the field of gastric medicine, can solve the problems of long antacid action time, failure to solve the disintegration time of aluminum magnesium carbonate, etc., and achieves uniform dispersion of the main drug, long antacid effect maintenance time, The effect of short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: per 1000 dosage
[0030]
[0031]Preparation method: Weigh micronized aluminum magnesium carbonate, 50% croscarmellose sodium, xylitol, microcrystalline cellulose, citric acid, sodium lauryl sulfate, sodium saccharin, mix well, pass 120 mesh screen. Use 80% ethanol solution to make polyvinylpyrrolidone (K90) into ethanol solution with a concentration of 2%, add it to the above powder, use high-efficiency wet granulation mechanism soft material, and pass the obtained particles through a 24-mesh sieve at 50 ° C. Dry, granulate with 24 meshes, add remaining 50% croscarmellose sodium, menthol, magnesium stearate, talcum powder. Mix evenly, and press into tablets with a weight of about 1g per tablet.
Embodiment 2
[0032] Embodiment 2: per 1000 dosage
[0033]
[0034] Preparation method: Weigh micronized aluminum magnesium carbonate, 50% low-substituted hydroxypropyl cellulose, mannitol, precrossified starch, maleic acid, sodium lauryl sulfate, steviol glycoside, mix well, pass through 150 mesh sieve. Prepare hypromellose (E50) into a 10% aqueous solution, add it to the above-mentioned powder, use a high-efficiency wet granulation mechanism to make the soft material, pass the granules through a 30-mesh sieve, dry at 60°C, and dry them with a 30-mesh sieve. granules, add the remaining 50% of the amount of low-substituted hydroxypropyl cellulose, magnesium stearate, menthol. Mix evenly, and press into tablets with a weight of about 1.5g per tablet.
Embodiment 3
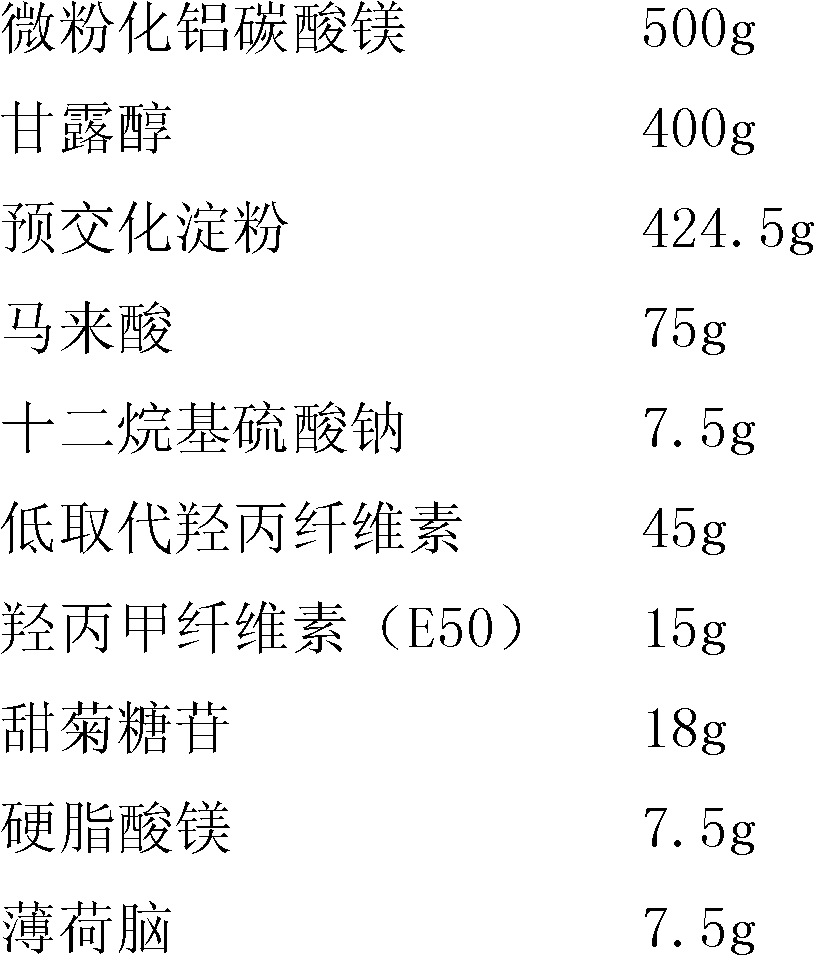
[0035] Embodiment 3: per 1000 dosage
[0036]
[0037]
[0038] Preparation method: Weigh micronized aluminum magnesium carbonate, 50% low-substituted hydroxypropyl cellulose, croscarmellose sodium, lactose, microcrystalline cellulose, tartaric acid, steviol glycoside, mix well, pass through 120 mesh sieve. Use 30% ethanol solution to prepare polyvinylpyrrolidone (K30) into a 6% ethanol solution, add it to the above powder, use a high-efficiency wet granulation mechanism to make the soft material, and pass the prepared granules through a 30-mesh sieve. Dry, granulate with 30 meshes, add the remaining 50% low-substituted hypromellose, croscarmellose sodium, magnesium stearate, silicon dioxide, and menthol. Mix evenly, and press into tablets with a weight of about 0.7g per tablet.
[0039] Sample acid test
[0040] Inspection method: take a few pieces of this product, grind them finely, take an appropriate amount of fine powder, approximately equivalent to 0.5g of alumi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com