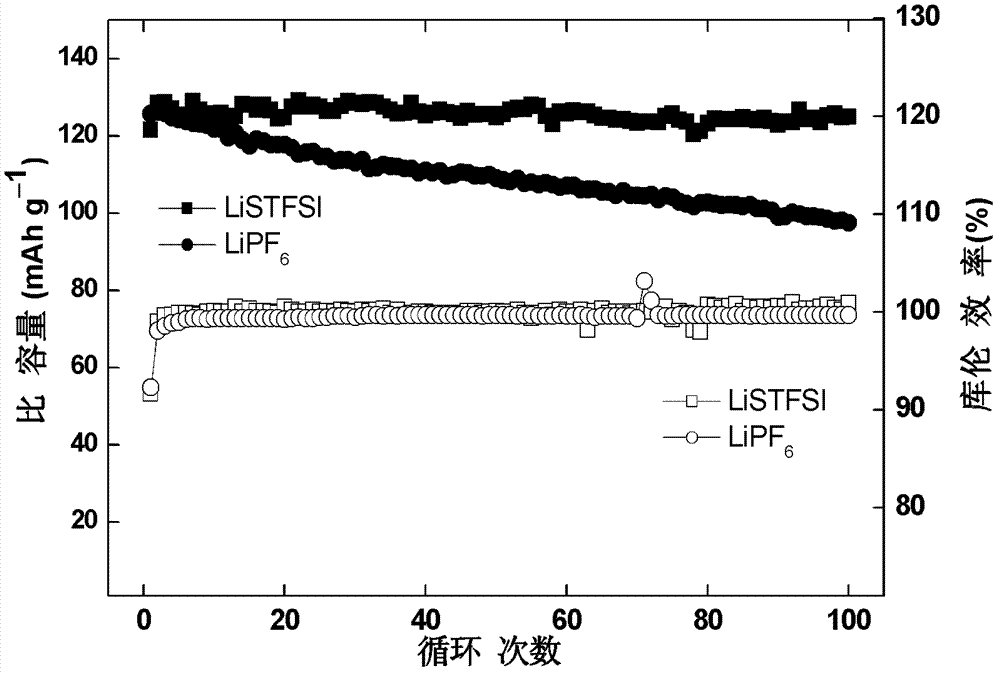
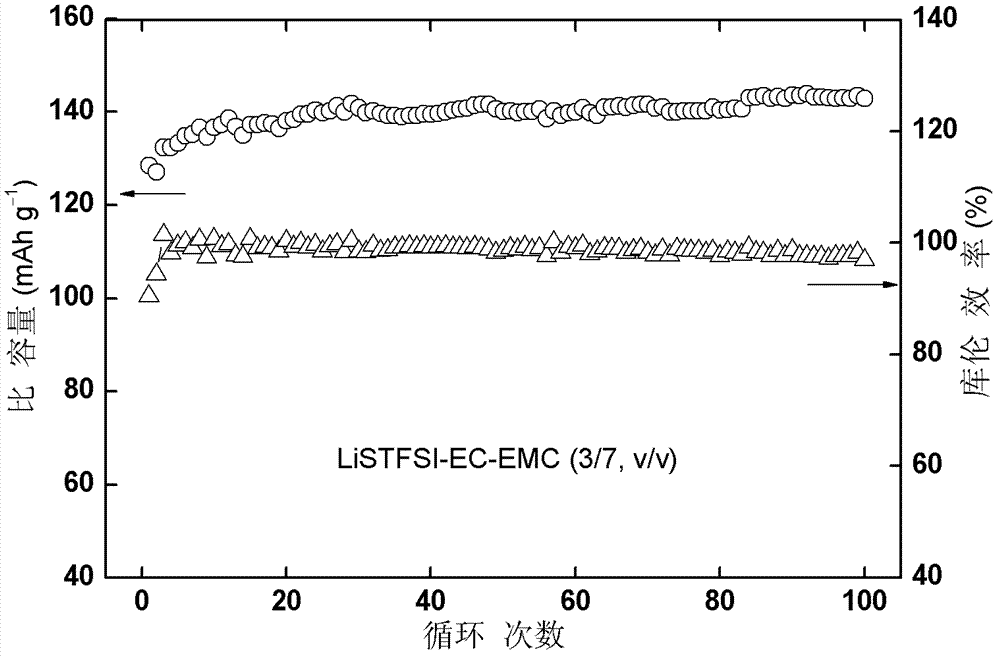
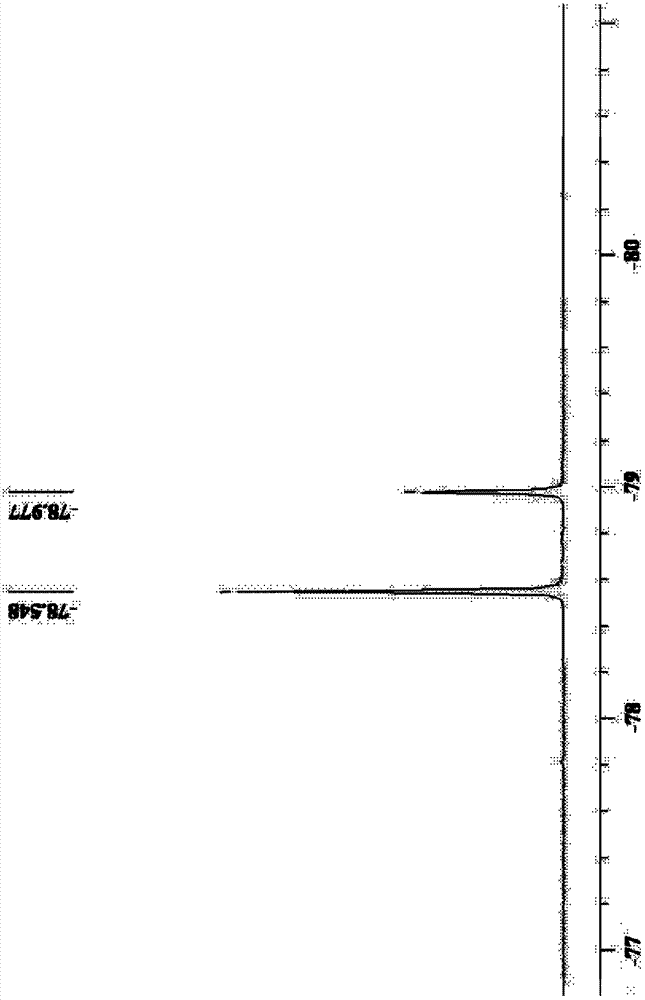
Imine alkali metal salt and ion liquid and application of same as non water electrolyte
A technology of alkali metal salts and imine bases, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of long synthesis route, high price, and unsuitable for large-scale preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0115] Embodiment 1-9 relates to the preparation of imide alkali metal salt containing "S-fluoroalkylsulfonylimide group"
Embodiment 1
[0116] Embodiment 1: (trifluoromethylsulfonyl) (trifluoromethylsulfinyl) potassium imide ([(CF 3 SO 2 )(SOCF 3 )N] K) The preparation synthesis reaction scheme is as follows:
[0117] CF 3 SO 2 Na+SOCl 2 → CF 3 SOCl+NaCl+SO 2
[0118]
[0119] Add sodium trifluoromethanesulfinate (CF 3 SO 2 Na) (65.5 g, 0.42 mol), 100 mL of chlorobenzene. Stir mechanically, heat to 30°C, slowly add SOCl dropwise 2 (59.5 g, 0.5 mol), stirring was continued for 2 hours after the dropwise addition was completed. Atmospheric distillation, collecting fractions at 30°C. 55 g of trifluoromethanesulfonyl chloride (pale brown liquid) are obtained, yield 87%.
[0120] Add trifluoromethanesulfonamide (CF 3 SO 2 NH 2 ) (29.8g, 0.2mol), acetonitrile (80mL), and pyridine (31.6g, 0.4mol) were used as acid-binding agents, stirred and dissolved, and trifluoromethylsulfinyl chloride (CF 3 SOCl) (35g, 0.23mol), the ice bath was removed after the dropwise addition, and after stirring at room t...
Embodiment 2
[0121] Example 2: Trifluoromethyl (S-trifluoromethylsulfonimide) sulfonamide (CF 3 (CF 3 SO 2 N)SONH 2 ) preparation
[0122]
[0123] Potassium (trifluoromethylsulfonyl) (trifluoromethylsulfinyl)imide ([(CF 3 SO 2 )(SOCF 3 )N]K) (66.7g, 0.22mol) was dissolved in 200mL distilled water, and was added successively under mechanical stirring 2 OSO 3 H) (38.2g, 0.34mol) and sodium acetate (CH 3 CO 2 Na) (27.9g, 0.34mol), after stirring for 8 hours, extracted with ether (50mLx3), and combined the organic phases. After adding anhydrous magnesium sulfate to dry, filter, collect the filtrate, and remove ether by rotary evaporation to obtain a white flaky solid. Yield 34.8g, 60% yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com