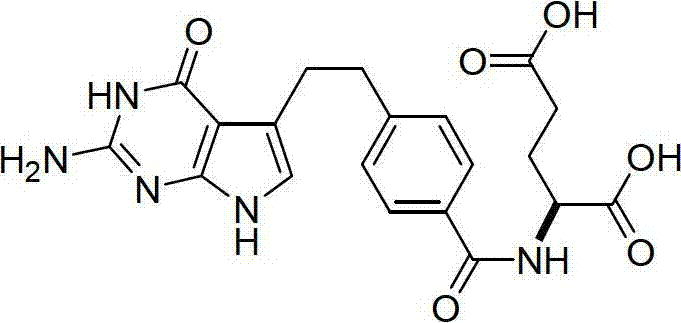
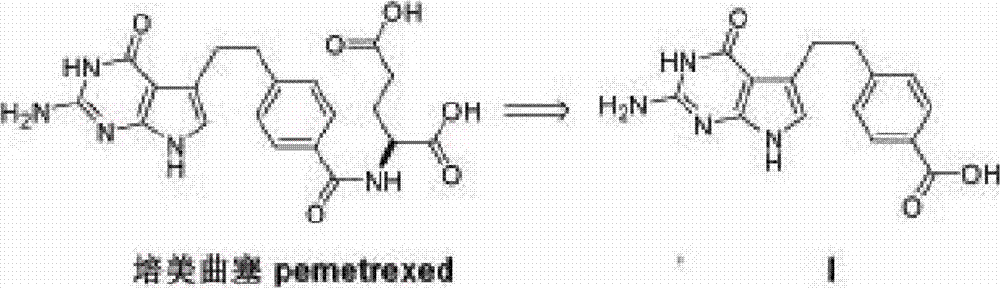
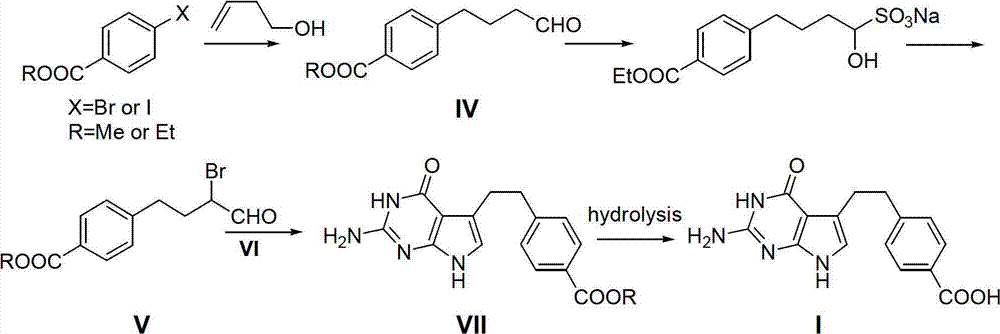
Preparation method of pemetrexed intermediate
A technology for pemetrexed and intermediates, which is applied in the field of preparation of pemetrexed intermediates, can solve the problems that the preparation method is not simple and convenient enough, the production cost is high, and the classical stability is not enough, and achieves easy operation, reduced manufacturing steps, The effect of promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of compound 4-phenyl-1-butanol II:
[0044] Add dry benzene 250mL in 1L dry three-necked flask, start stirring, slowly add aluminum trichloride (80.0g, 0.63mol), under the control temperature 10-15 ℃, dropwise add 4-chloro-1-butanol ( 60.0 g, 0.61 mol), the addition was completed within about 1 hour. The stirring reaction was continued at this temperature for 6 hours. The reaction solution was poured into 200 mL of ice water, stirred for 15 minutes, and the solid phase residue was removed by filtration. Let stand to layer. The organic phase was separated, the solvent benzene was distilled off under normal pressure, and then the solvent was distilled off under reduced pressure to obtain 71.6 g of colorless transparent liquid 4-phenyl-1-butanol II, with a yield of 78.3%.
Embodiment 2
[0045] Embodiment two, the preparation of compound 4-(4-methoxycarbonylphenyl)-1-butanol III:
[0046] Under nitrogen protection, add 250 mL of solvent tetrahydrofuran into a 1 L dry three-necked flask, start stirring, and then add aluminum trichloride (66.0 g, 0.5 mol), boron trifluoride (3.4 g, 0.05 mol) and 4-phenyl - 1-Butanol II (75.0 g, 0.5 mol). Under controlled temperature of 0-5°C, methyl chloroformate (56.4 g, 0.60 mol) was added dropwise, and the addition was completed within about 1 hour. Warm up to 15-20°C and continue stirring for 12 hours. The reaction solution was poured into 200 mL of ice water, stirred for 15 minutes, and the solid phase residue was removed by filtration. Extracted three times with 250 mL, 200 mL and 200 mL of ethyl acetate successively, combined the organic phases, washed with 10% sodium bicarbonate solution and 200 mL of pure water each, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obta...
Embodiment 3
[0047] Embodiment three, the preparation of compound 4-(4-methoxycarbonylphenyl)-1-butyraldehyde IV:
[0048] Under the protection of nitrogen, add 500mL of solvent dichloromethane and 4-(4-methoxycarbonylphenyl)-1-butanol III (52.0g, 0.25mol) into a 1L three-necked flask, start stirring, and keep at 0-5° C, pyridinium chlorochromate (PCC) oxidizing agent (107.8 g, 0.5 mol) was added in 10 portions. Warm up to room temperature, stir for 4 hours, add 200 mL of ice water, stir for 15 minutes, and filter to remove solid impurities. The filtrate was allowed to stand for layers, and the organic phase was separated. The organic phase was washed with 200 mL of 2% sodium bicarbonate solution and 200 mL of pure water, dried over anhydrous magnesium sulfate, and decolorized on silica gel. Concentration under reduced pressure gave 4-(4-methoxycarbonylphenyl)-1-butyraldehyde IV as a yellow oil, 41.8 g, yield 80.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com