Drug combination containing borneol
A composition and drug technology, applied in the field of medicine, can solve the problems of variable content of active ingredients, volatile, affecting curative effect, etc., and achieve the effects of good therapeutic effect, controllable quality and clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
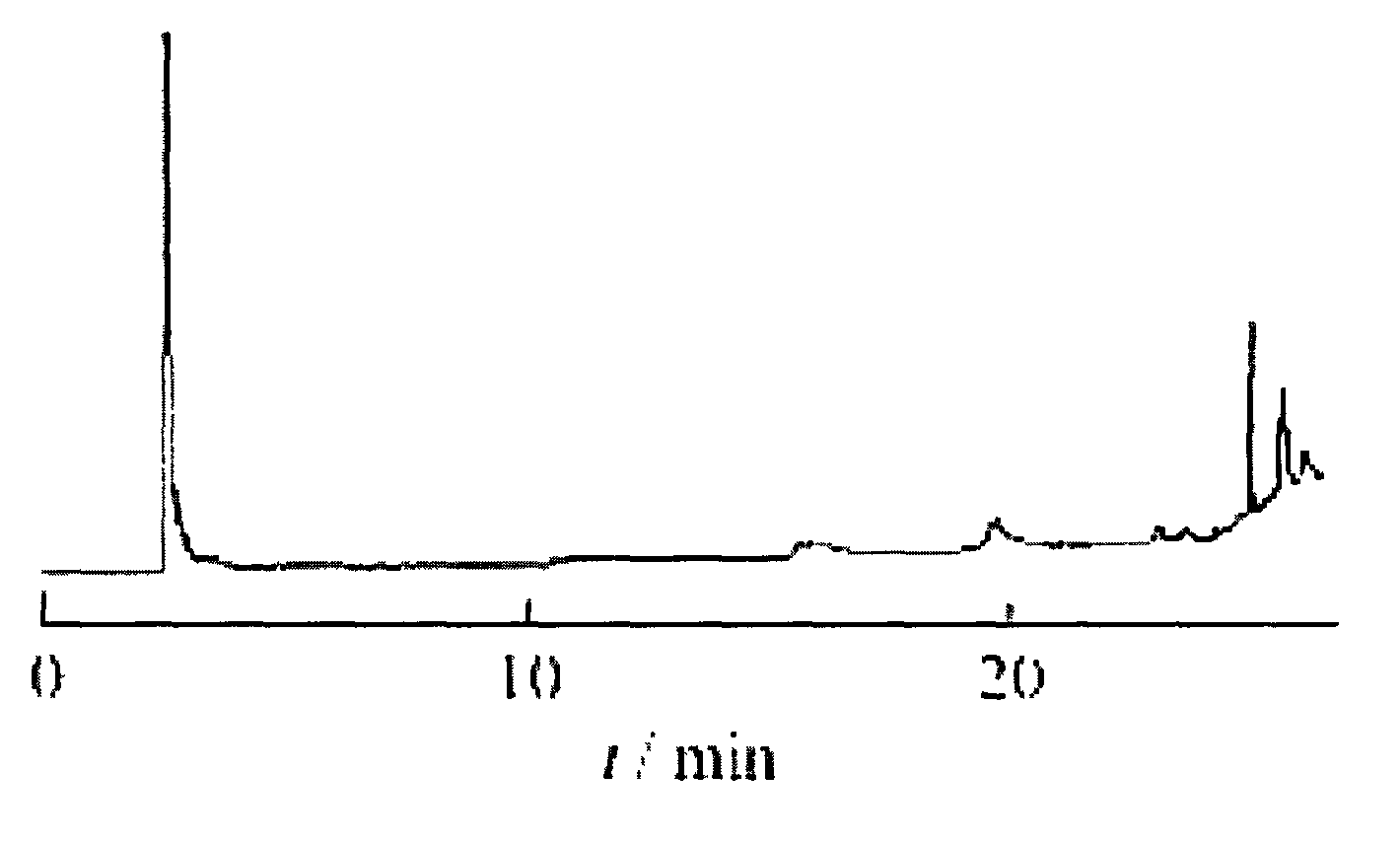
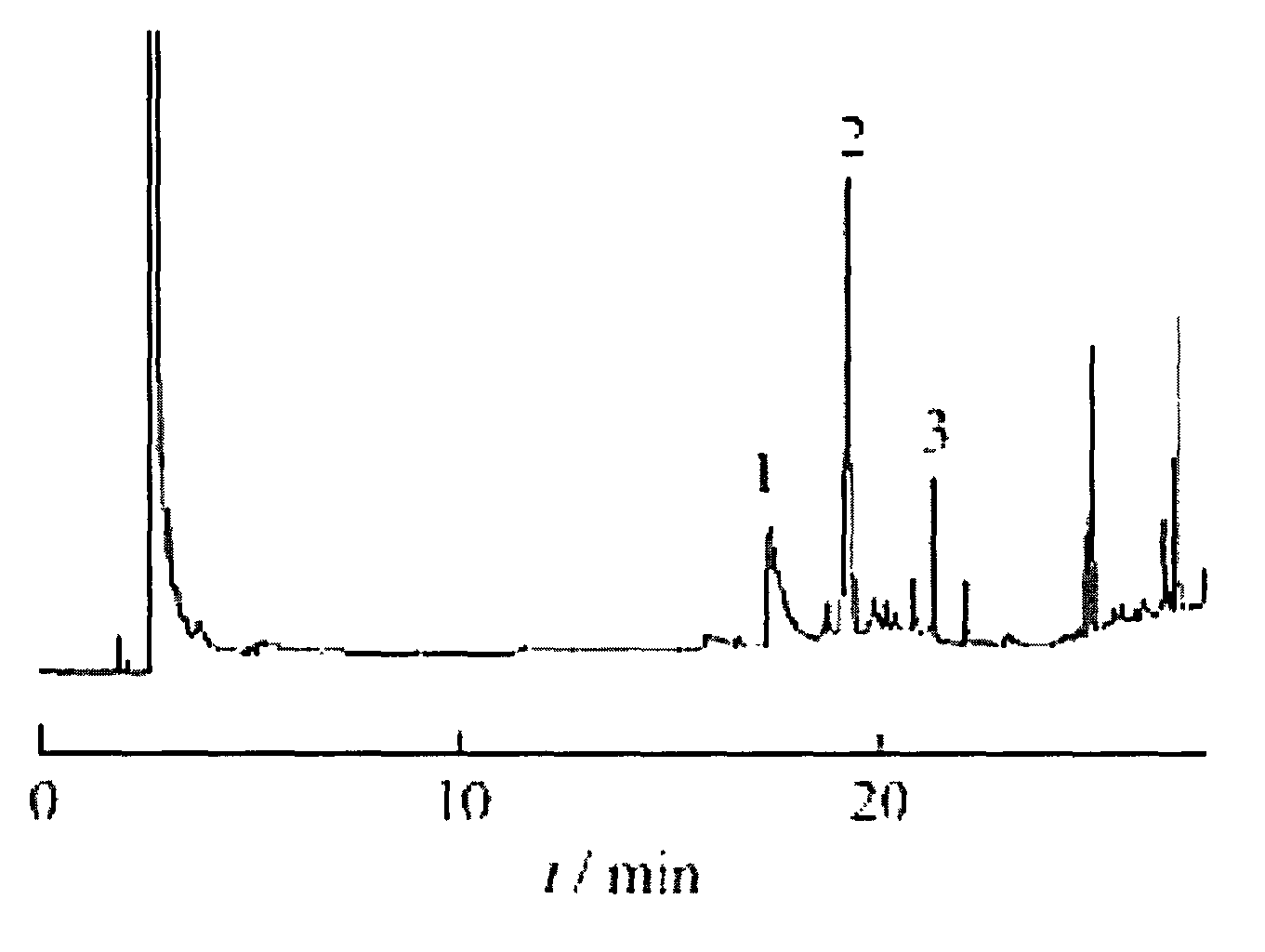
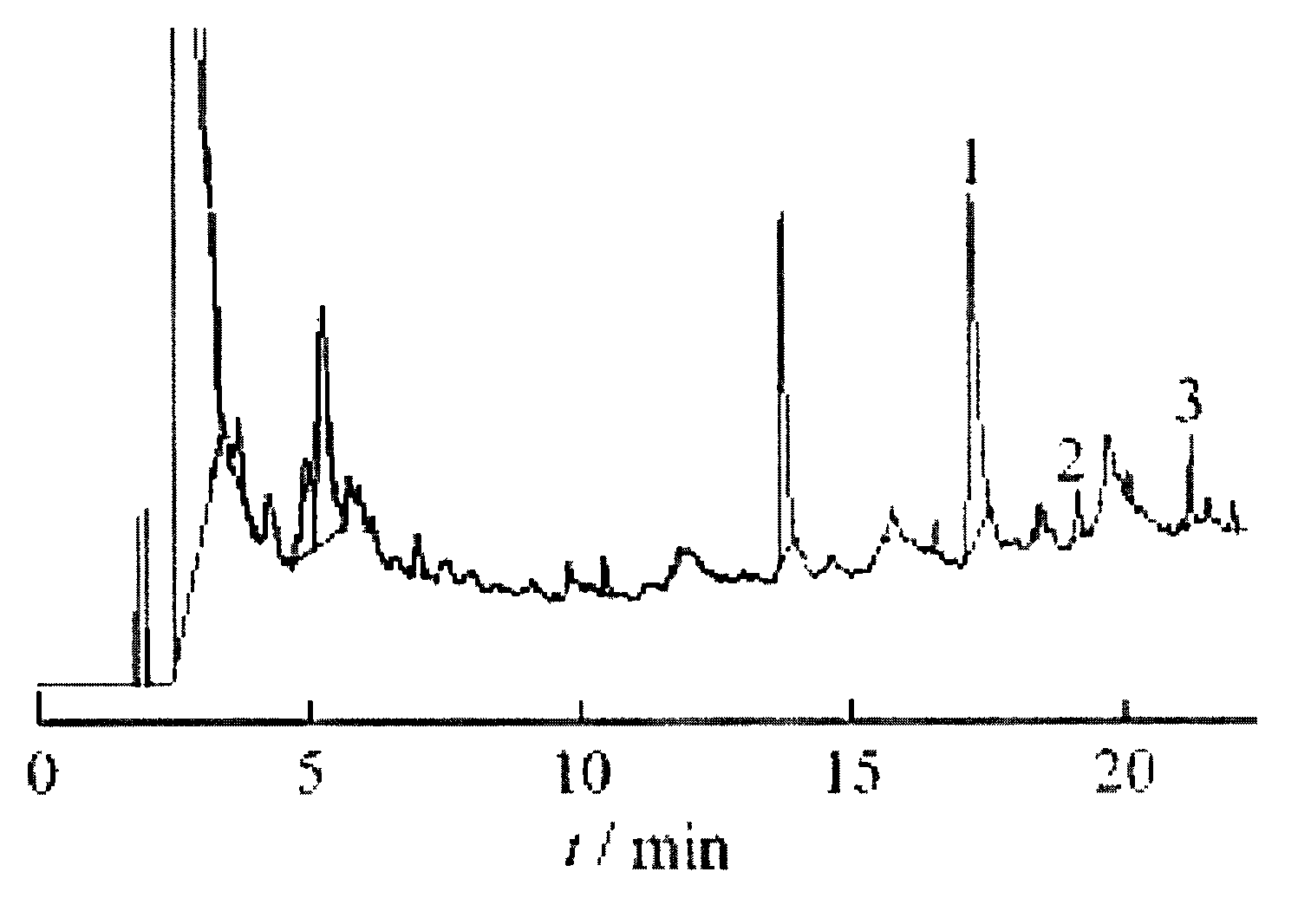
Image
Examples
Embodiment 1
[0172] Prescription: gemmaconone 13.9g, furadienes 39.4g, curdione 24g, β-elemene 9.7g, curcumol 0.5g, curcumene 0.5g, borneol 75g (the content of borneol in borneol is 90.0%) .
[0173] Dosage form: suppository.
[0174] Preparation method: mix the prescribed amount of gemmacone, furadienes, curdione, β-elemene, curcumol, curcumene with 70g of polysorbate 80, dissolve 75g of borneol with an appropriate amount of ethanol, and mix with the above solution Mix well.
[0175] The polyoxyethylene stearate 1524g of recipe quantity is put on the water bath and is heated and melted, adds above-mentioned medicinal liquid, stirs, filling, makes 1000 suppositories, each 1.74g.
Embodiment 2~8
[0177] Prepare the suppository according to the following prescription, the preparation method is the same as in Example 1, (the content of borneol in the borneol is 95.5%).
[0178] Example
[0179] β-Elemene
Embodiment 9
[0181] Prescription: Gemacerone 6.5g, Furandiene 32g, Curdione 21g, β-Elemene 8.5g, Curcumol 0.45g, Borneolum 35g (the content of borneol in the Borneolum is 96.3%).
[0182] Dosage form: effervescent tablet.
[0183] Preparation:
[0184] 1) Take β-cyclodextrin, add appropriate amount of water according to 25ml / g, stir to make it fully dissolved, and heat if necessary to obtain β-cyclodextrin solution; ene, curcumol, borneol 35g, diluted with absolute ethanol, slowly added to the β-cyclodextrin solution, continued to stir until a homogeneous phase was formed, placed in the refrigerator overnight, filtered with suction, washed 3 times with a small amount of petroleum ether, frozen, A white powder was obtained.
[0185] 2) Mix the above-mentioned white powder with 35g of borneol, 150g of citric acid and 160g of lactose; take another 120g of sodium bicarbonate and 160g of lactose, mix them evenly, and use 5% PVP absolute ethanol solution as a binder to granulate, at 50°C Dry,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com