Recombinant human apoE peptide mimics, preparation method and application
A peptide segment and binding region technology, applied in the field of the preparation of apolipoprotein E peptidomimetics, can solve the problems of inter-immunogenicity and safety, organ specificity, low solubility, cytotoxic fragments, complex structure, etc. The effect of chemical expression and purification, high yield, and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Design of recombinant vector and purification and identification of EpK peptide
[0061] The present invention designs a derivative peptide (EpK) containing an N-terminal LDLR binding region and a C-terminal lipid-binding region of human apoE. This peptide contains a cysteine (Cys) at the N-terminal and an apoE LDLR-binding region (the LRKLRKRLLR peptide segment at positions 141-150), followed by an apoE lipid binding region (the QVAEVRAKLEEQAQQIRLQAE peptide segment at positions 234-254) connected by 6 Lys residues.
[0062] The 38 amino acid sequence of EpK is as follows:
[0063] NH 2 -CLRKLRKRLLRKKKKKKQVAEVRAKLEEQAQQIRLQAE-COOH
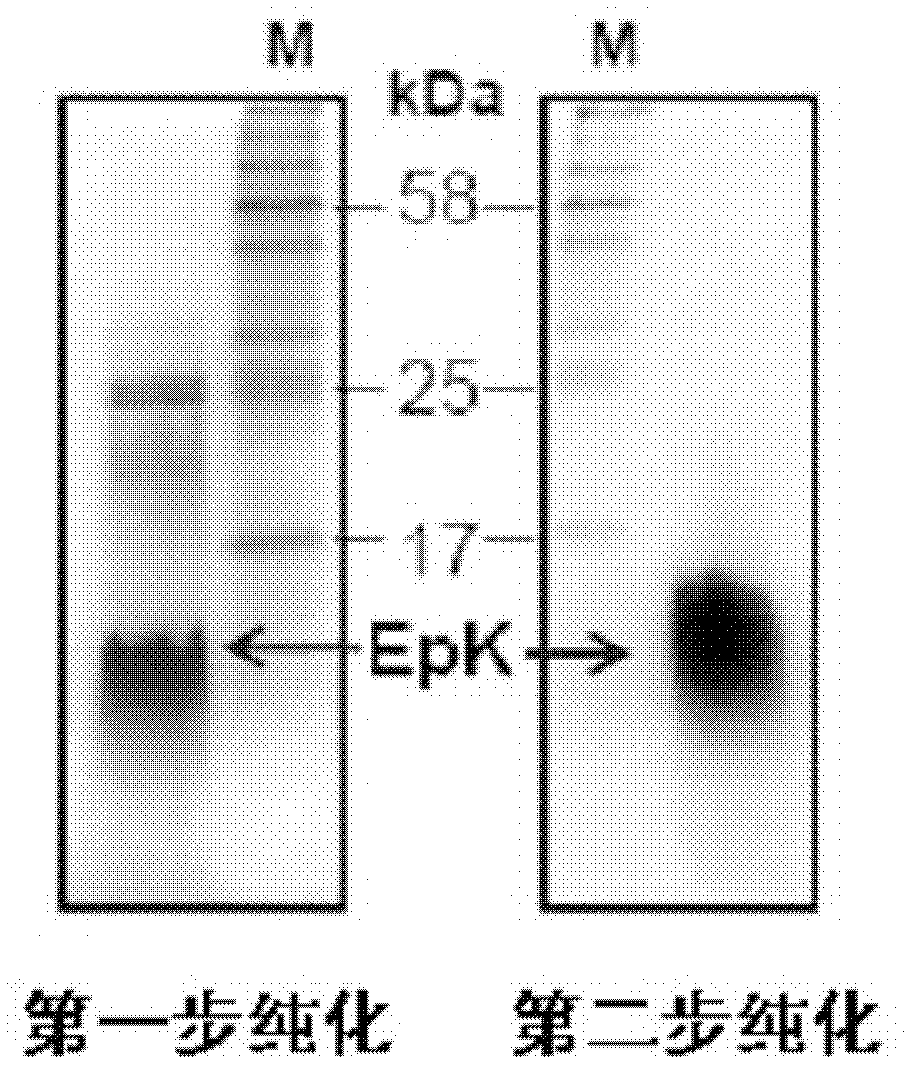
[0064] The present invention uses genetic engineering technology to construct a recombinant vector, and the recombinant peptide expressed in bacteria undergoes two-step affinity chromatography of chitin beads and heparin Sepharose CL-6B to obtain a purified recombinant human apoE peptidomimetic (EpK). The specific steps are a...
Embodiment 2
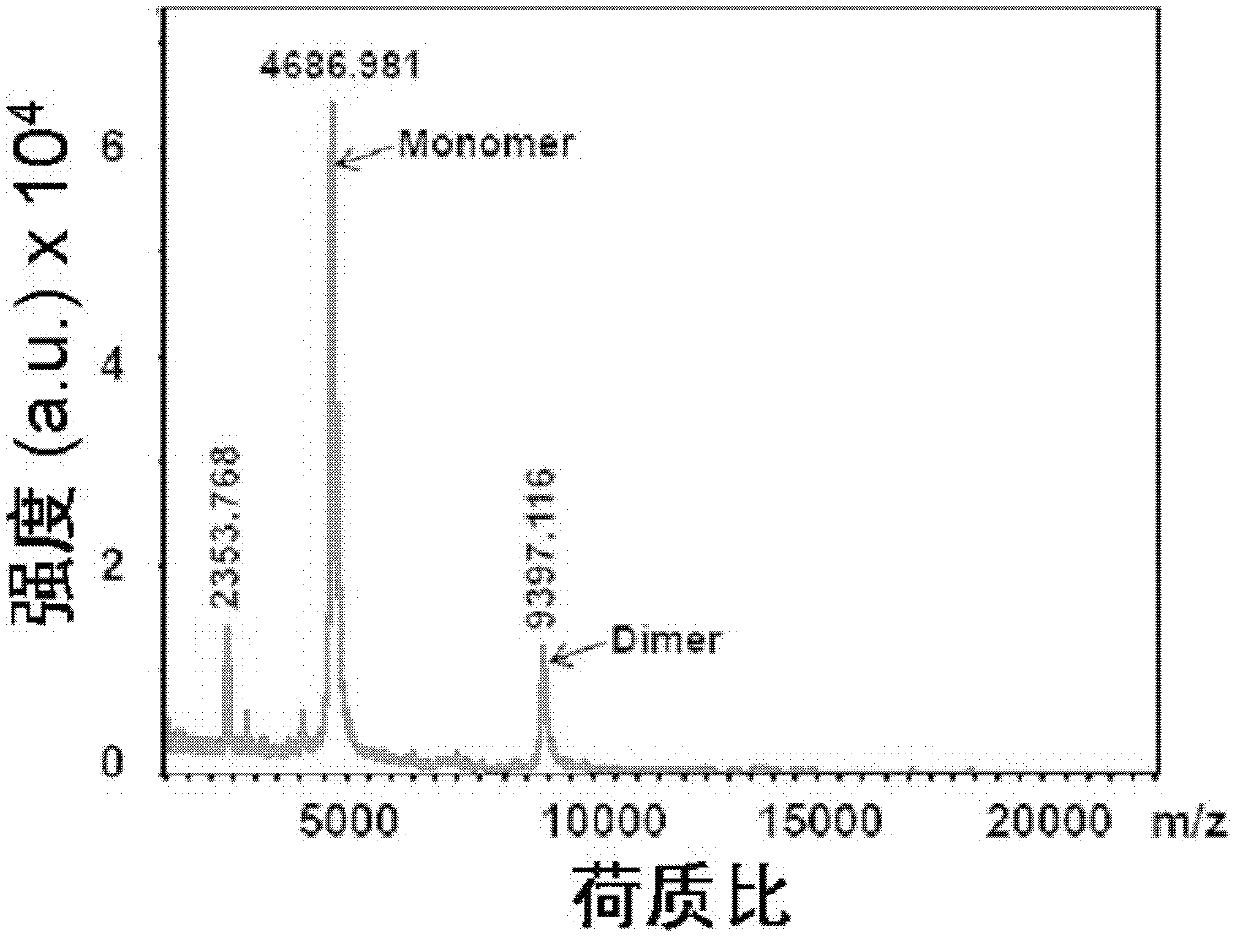
[0076] Example 2: Characteristic detection of purified EpK peptide secondary structure and lipid-binding activity
[0077] ApoE has a very high α-helical structure. According to the designed EpK peptide sequence, it contains a part of the LDLR binding region and a lipid binding region of human apoE. Based on the prediction of the protein secondary structure prediction software (Network Protein Sequence Analysis), EpK may contain 89.74% In order to confirm the ability of EpK to form an α-helix, we analyzed the secondary structure of EpK by circular dichroism, and compared EpK with human full-length apoE3 by DMPC binding experiments to observe their binding to lipids. ability.
[0078] (1) Characterization of the secondary structure of the EpK peptide
[0079] Circular dichroism (CD) was used to measure the secondary structure of the EpK peptide, that is, at 37°C, a cuvette with a liquid layer thickness of 0.2cm was used to measure the spectrum of the sample in the far ultravio...
Embodiment 3
[0088] Example 3: EpK enhances macrophage cholesterol efflux and inhibits macrophage inflammation
[0089] Functional evaluation of purified EpK using primary mouse peritoneal macrophages. Examination of whether EpK mediates cholesterol efflux in cholesterol-loaded macrophages. Second, to detect whether EpK inhibits primary apoE - / - Inflammatory responses induced by LPS in mouse peritoneal macrophages. apoE - / - The mouse model (from Jackson Laboratory) was raised in the Animal Experiment Center (SPF grade) of Zhongnan Hospital of Wuhan University.
[0090] (1) Cell culture:
[0091] In order to obtain mouse macrophages, mice were injected intraperitoneally with 3ml 3% thioglycollate, and mice were euthanized 3 days later, and mouse peritoneal macrophages were collected by perfusion with 10ml PBS. Cells were seeded in 6-well or 24-well cell culture plates, cultured in Dulbecco's Modified Eagle Medium (DMEM) (both purchased from GIBCO) containing 10% fetal bovine serum (FBS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com