Method for measuring concentration of enantiomer of ibuprofen in chiral liquid-liquid extraction water phase by using high performance liquid chromatography (HPLC)
A technology of enantiomer and water extraction, which is applied in the field of determination of ibuprofen enantiomer concentration in the aqueous phase solution of chiral liquid-liquid extraction system, which can solve the problems of inability to achieve baseline separation, failure to meet analysis requirements, high price of n-hexane, etc. problem, achieve the effect of ensuring stability, avoiding baseline shift, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
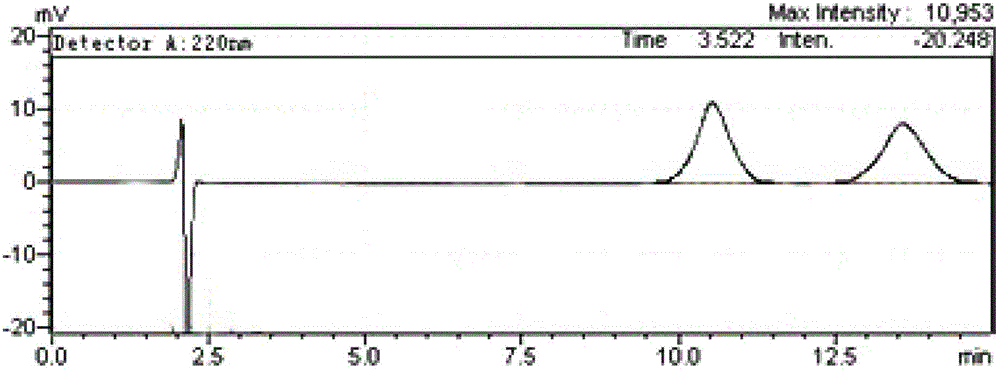
[0044] Prepare the sodium phosphate-phosphoric acid solution of 0.08g / L ibuprofen racemate and the n-octane solution of L-n-pentyl tartrate, complete the chiral liquid-liquid extraction experiment, get the aqueous phase ibuprofen solution after the liquid separation, and use 0.45μm microporous membrane filtration.
[0045] use alpha 1 - Chiral chromatographic column with acidic glycoprotein as filler, the mobile phase is methanol (2%)-sodium phosphate / phosphoric acid (pH=7.0), the flow rate is 0.5ml / min, the detection wavelength is 220nm, and the injection volume is 20μL. Obtain the sample analysis spectrum. The separation degree of the two enantiomers of ibuprofen was 1.512, reaching the baseline separation, and the peak shape was good, which realized the analysis of the two enantiomers of ibuprofen in the aqueous phase solution of the chiral liquid-liquid extraction experiment.
Embodiment 2
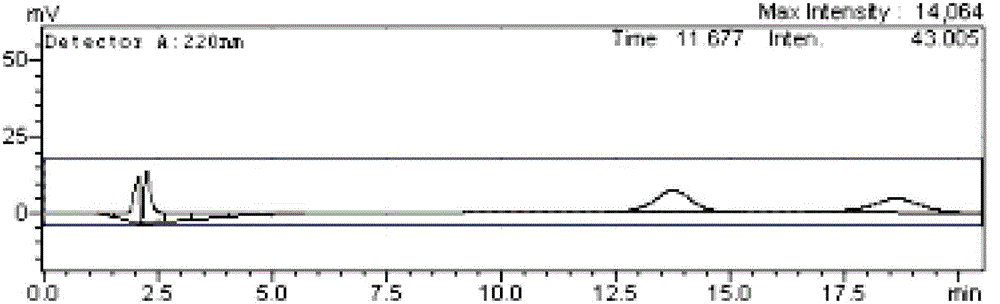
[0047] Prepare a 0.02 g / L sodium phosphate-phosphoric acid solution of ibuprofen racemate, and filter it with a 0.45 μm microporous membrane.
[0048] use alpha 1 -Acid glycoprotein AGP chiral chromatography column, the mobile phase is methanol (0.5%)-sodium phosphate / phosphoric acid (pH=6.5), the flow rate is 0.5ml / min, the detection wavelength is 220nm, and the injection volume is 20μL. Obtain the sample analysis spectrum. Compared with Example 1, the retention times of the two enantiomers of ibuprofen become 13.752min and 18.762min respectively, which is larger than the retention time of 2% methanol volume fraction, and the separation degree is increased to 1.825, which can reach the baseline separate.
Embodiment 3
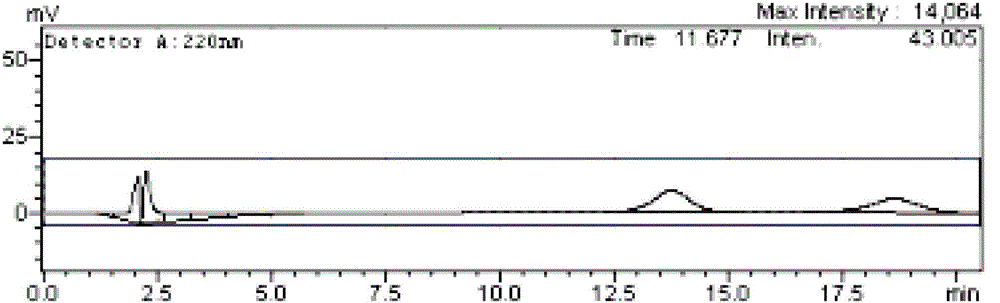
[0050] Prepare a 0.0006 g / L sodium phosphate-phosphoric acid solution of ibuprofen racemate, and filter it with a 0.45 μm microporous membrane.
[0051] use alpha 1 -Acid glycoprotein AGP chiral chromatography column, the mobile phase is methanol (2%)-sodium phosphate / phosphoric acid (pH=7.0), the flow rate is 0.3ml / min, the detection wavelength is 230nm, and the injection volume is 20μL. Obtain the sample analysis spectrum. The resolution of the two enantiomers of ibuprofen is 1.822, which can achieve baseline separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com