Method for synthesizing light color low-chlorinity o-cresol-formaldehyde epoxy resin
A novolac epoxy resin and synthesis method technology, applied in the field of organic polymer compound synthesis, can solve the problems of high loss of epichlorohydrin, not strict equipment requirements, poor color of products, etc., and achieve the effect of reducing side reactions and reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
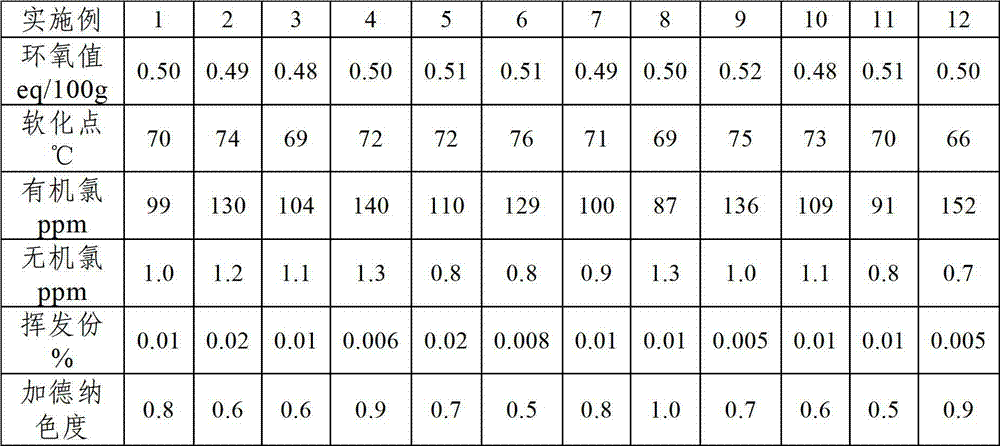
Examples
Embodiment 1
[0025] Step 1, add o-cresol aldehyde resin 100g, organic solvent methyl isobutyl ketone 500g in reaction kettle, obtain o-cresol aldehyde resin solution, then add epichlorohydrin 150g and reducing agent zinc powder 5g in o-cresol aldehyde resin solution , heating up to 80°C for reduction decolorization reaction for 0.5h;
[0026] Step 2. Under vacuum conditions, add 5 g of phase transfer catalyst benzyltriethylammonium chloride (TEBA) and 3 g of cocatalyst sodium hydroxide to the reaction system after the reduction decolorization reaction in step 1, and the pressure is 900 mbar. Etherification reaction at 80°C for 3 hours to obtain etherification reaction product;
[0027] Step 3. Under vacuum conditions, add 45 g of NaOH solution with an alkali catalyst mass concentration of 55% to the etherification reaction product described in step 2, and perform a ring-closure reaction at a pressure of 900 mbar and a temperature of 90° C. for 8 h;
[0028] Step 4, filter the reaction sys...
Embodiment 2
[0030] The present embodiment is the same as Example 1, except that the organic solvent is 250 g each of methyl isobutyl ketone and cyclohexanone; the reducing agent is 3 g of Zn powder and 2 g of Al powder; the phase transfer catalyst Be tetrabutylammonium bromide 2g, benzyltriethylammonium chloride 3g; Described promotor is sodium hydroxide and potassium hydroxide total 3g, wherein sodium hydroxide and potassium hydroxide mass ratio 1: 1; Described The alkali catalyst is 45 g of a mixture solution of 55% sodium hydroxide and potassium hydroxide, wherein the mass ratio of sodium hydroxide to potassium hydroxide is 1:1.
Embodiment 3
[0032] Step 1, add o-cresol novolac resin 100g, organic solvent toluene 700g in reaction kettle, obtain o-cresol novolac resin solution, then add epichlorohydrin 200g and reducing agent zinc powder 2g in o-cresol novolac resin solution, be warming up to 50 ℃ reduction decolorization reaction 1h;
[0033] Step 2. Under vacuum conditions, add 0.5 g of phase transfer catalyst tetrabutylammonium bromide and 1 g of cocatalyst potassium hydroxide to the reaction system after the reduction decolorization reaction in step 1. The pressure is 900 mbar and the temperature is 80 ° C. Under the etherification reaction for 7h, the etherification reaction product was obtained;
[0034] Step 3. Under vacuum conditions, add 60 g of ammonium hydroxide solution with an alkali catalyst mass concentration of 50% to the etherification reaction product described in step 2, and perform a ring-closure reaction at a pressure of 400 mbar and a temperature of 80° C. for 10 h;
[0035]Step 4, filter the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com