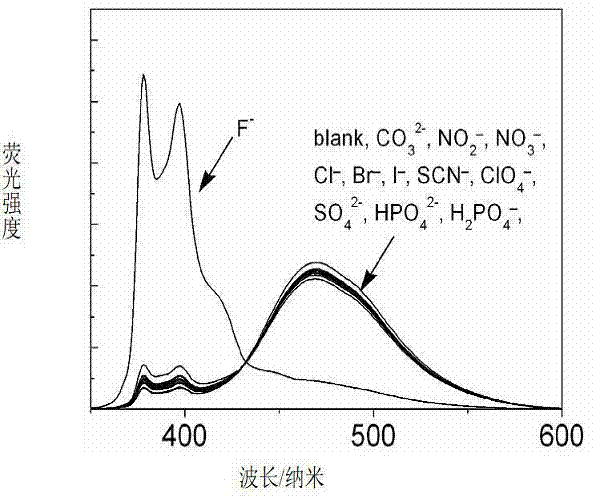
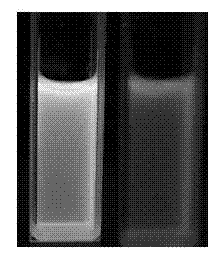
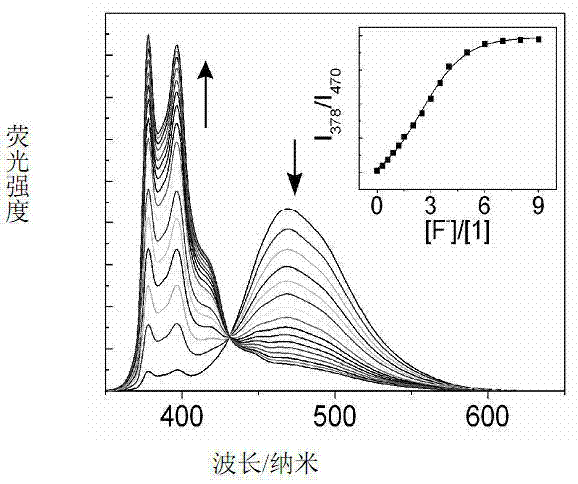
Metering type fluorinion fluorescence probe and preparation method
A fluorescent probe, fluoride ion technology, applied in biochemical equipment and methods, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problem of inability to complete fluoride ion detection, and achieve excellent selectivity, sensitivity, and yield. High and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a (200 mL) round bottom flask, add pyrenemethanol (2 g, 8.6 mmol), under nitrogen protection, add 90 mL of tetrahydrofuran and triethylamine (2.64 mL, 18.9 mmol), and then add 1,1 , 2,2-Tetramethyldichlorodisilane (810 μL, 4.3 mmol), about 20 minutes, keep the reaction for 10 hours after the dropwise addition is completed. Then directly remove the precipitate by suction filtration, extract the filtrate three times with dichloromethane, wash three times with water, dry with anhydrous sodium sulfate, and separate with a chromatographic column (petroleum ether as eluent) to obtain the pure product 1 as a white solid, namely Quantitative fluoride ion fluorescent probe 1 (1.92 g) with the structural formula (A1), the yield was 77.3%.
[0031] 1 H NMR (400 MHz, CDCl 3 ) δ: 8.15-8.04(m, 10H), 8.01-7.93(m, 6H), 7.85(d, 2H), 5.42(s, 4H), 0.37 (s, 12H)
[0032] HR-MS: Calcd. for C 38 h 34 o 2 Si 2 [M + ] 578.8464. Found 578.8468.
[0033] The synthetic reaction equat...
Embodiment 2
[0037] In a (200 mL) round bottom flask, add pyrenemethanol (2 g, 8.6 mmol), under nitrogen protection, add 55 mL of tetrahydrofuran and triethylamine (2.64 mL, 18.9 mmol), and then add dimethyl Dichlorosilane (800 μL, 4.3 mmol), about 30 minutes, keep the reaction for 8 hours after the dropwise addition is completed. Then direct suction filtration to remove a large amount of precipitate, the filtrate was extracted three times with dichloromethane, washed three times with water, dried with anhydrous sodium sulfate, and separated by chromatography column (petroleum ether as eluent) to obtain the pure product of white solid 2 , that is, the quantitative fluoride ion fluorescent probe 2 (1.82g) with the structural formula (A2), and the yield was 81.2%.
[0038] 1 H NMR (400 MHz, CDCl 3 ) δ: 8.15-8.05(m, 10H), 8.05-7.98(m, 6H), 7.89(d, 2H), 5.40(s, 4H), 0.39 (s, 6H)
[0039] HR-MS: Calcd. for C 36 h 28 o 2 Si [M + ] 520.6918. Found 520.6919.
[0040] The structural formula...
Embodiment 3
[0044] In a (200 mL) round bottom flask, add 10 mmol of pyrenyl n-butyl alcohol, add 100 ml of diethyl ether and triethylamine (3.07 mL, 22.5 mmol) under the protection of argon, and then add dimethyl dimethicone dropwise Chlorosilane 5 mmol, about 25 minutes, keep the reaction for 10 hours after the dropwise addition is completed, use the method of Example 1 to carry out suction filtration and purification, and obtain the pure product 3 of white solid, that is, the metering type fluoride ion fluorescence with the structural formula (A3) Probe 3 (2.43 g), yield 80.3%.
[0045] 1 H NMR (400 MHz, CDCl 3 ) δ: 8.18-8.10(m, 10H), 8.08-7.90(m, 6H), 7.86(d, 2H), 5.42(s, 4H), 3.17 (t, 8H), 1.60-1.90 (m, 8H) 0.39 (s, 6H)
[0046] HR-MS: Calcd. for C 42 h 40 o 2 Si [M + ] 604.8513. Found 604.8517.
[0047] The structural formula is:
[0048]
[0049] (A3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com