Lipid-based drug of serum degradable carrier and application method of lipid-based drug
A degradable, serum-based technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as variable configuration, high-level configuration disturbance, and loss of recognition ability. Achieve the effects of low immune response of blood cells, high mechanical strength and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
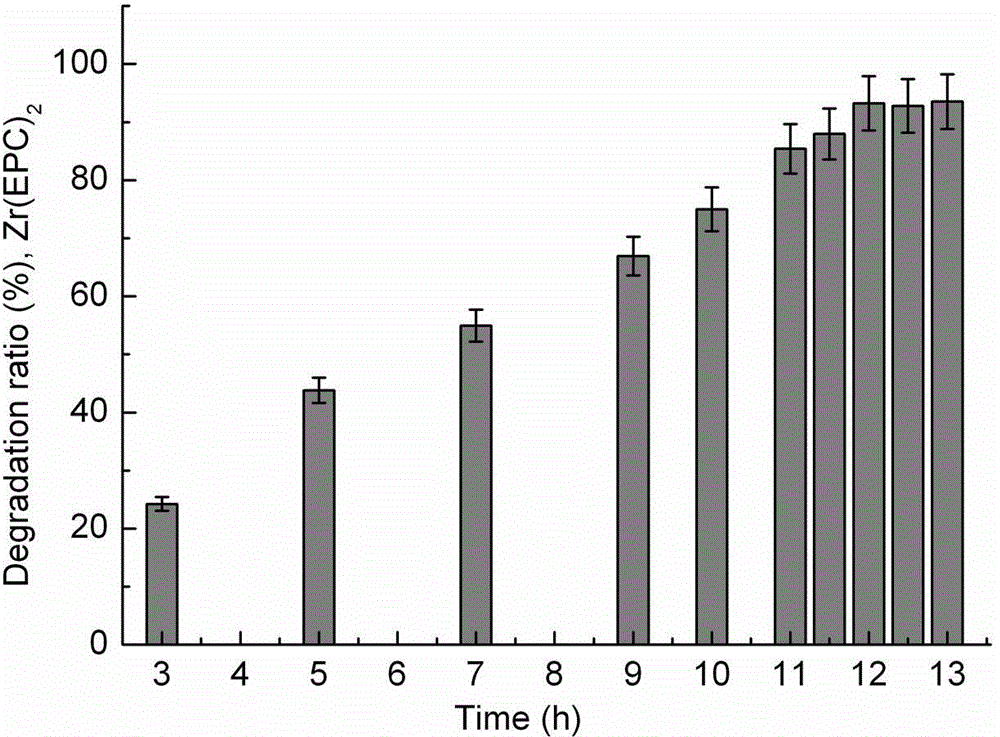
[0036] Zr(EPC) 2 Nanocarriers degrade in serum:
[0037] Accurately weigh 160 μg of dry carrier, add 1.5 ml of fresh serum, and stir at 3000 rpm / min for 20 min at 4 °C to obtain a translucent suspension dispersion, which is then shaken and degraded in a constant temperature oscillator at 37 °C; 3, 5, 7, 9, 10, 11, 11.5, 12, 12.5, 13 h Use a micropipette to draw 100 μl of degradation solution, centrifuge at 16000 rpm / min for 20 min at high speed; pipette 70 μl of supernatant in 1 ml colorimetric tube, dilute to 400 μl with distilled water, then add 200 μl ascorbic acid (100 g / l), 400 μl ammonium molybdate color solution (13 g ammonium molybdate + 0.35 g antimony potassium tartrate + 150 ml H 2 SO 4 500 ml); after 15 min of color development, the absorbance was measured at 700 nm, using serum samples as a reference.
[0038] The degradation rate of the carrier (%) = (the phosphorus mass of the supernatant ÷ the total phosphorus mass of the carrier) × 100 %.
[0039] Such as ...
Embodiment 2
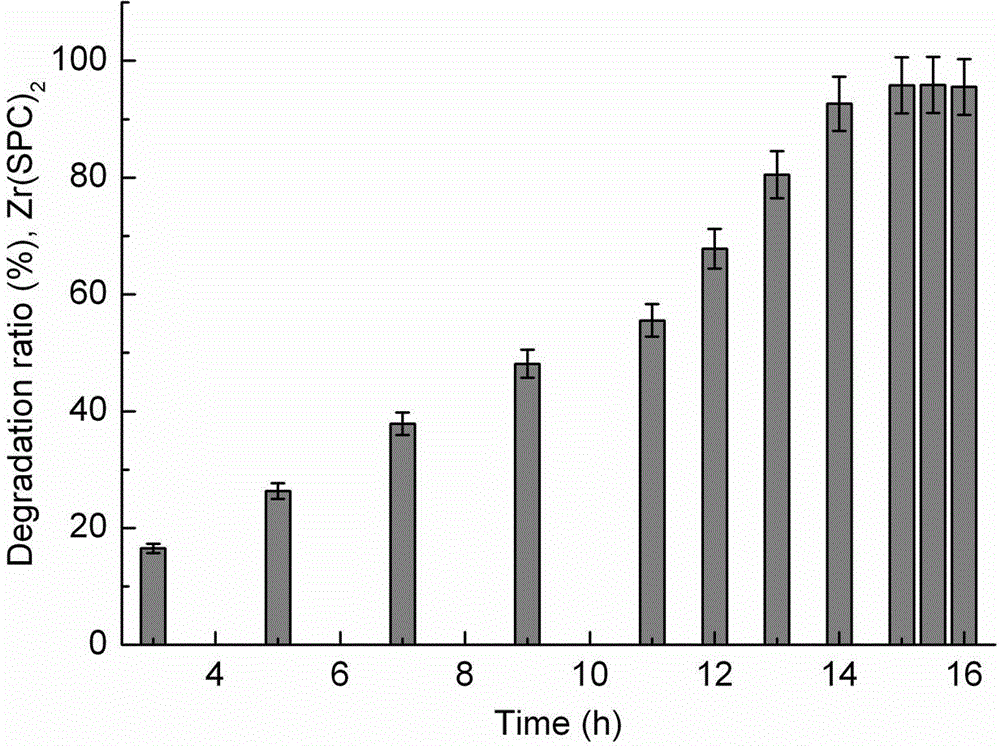
[0041] Zr(SPC) 2 Nanocarriers degrade in serum:
[0042] Add 160 μg of dry carrier into a centrifuge tube (2 ml) of 1.5 ml of fresh serum, stir at a high speed (3000 rpm / min) at 4°C, and turn into a translucent suspension dispersion after 20 min; place the centrifuge tube in 37°C constant temperature shaker, shake the degradation carrier; pipette 100 μl degradation solution at 3, 5, 7, 9, 11, 12, 13, 14, 15, 15.5, and 16 h respectively, and centrifuge at a high speed (16000 rpm / min) for 20 min; pipette 70 μl of the supernatant into a 1 ml colorimetric tube, dilute to a volume of 400 μl, add 200 μl of ascorbic acid (100 g / l), 400 μl of ammonium molybdate chromogenic solution (13 g ammonium molybdate + 0.35 g antimony potassium tartrate + 150 ml H 2 SO 4 500 ml), develop color for 15 min; measure the absorbance at 700 nm with reference to the serum sample, and calculate the mass of phosphorus and the degradation rate of the carrier. The degradation rate of the carrier (%) = ...
Embodiment 3
[0045] A serum-degradable lipid drug, comprising liposome and serum, wherein the liposome is formed by encapsulating the drug with lecithin zirconium nanoparticles, and the dosage ratio of liposome and serum is 187 μg: 1.5 mL. Lecithin zirconium nanoparticles are egg yolk phospholipid zirconium Zr (EPC) 2 , the drug is the ribosome inhibitory protein bitter melon MAP30.
[0046] The above-mentioned method of using the lipid drug of the serum-degradable carrier is to inject the carrier or the liposome drug into the serum in vitro.
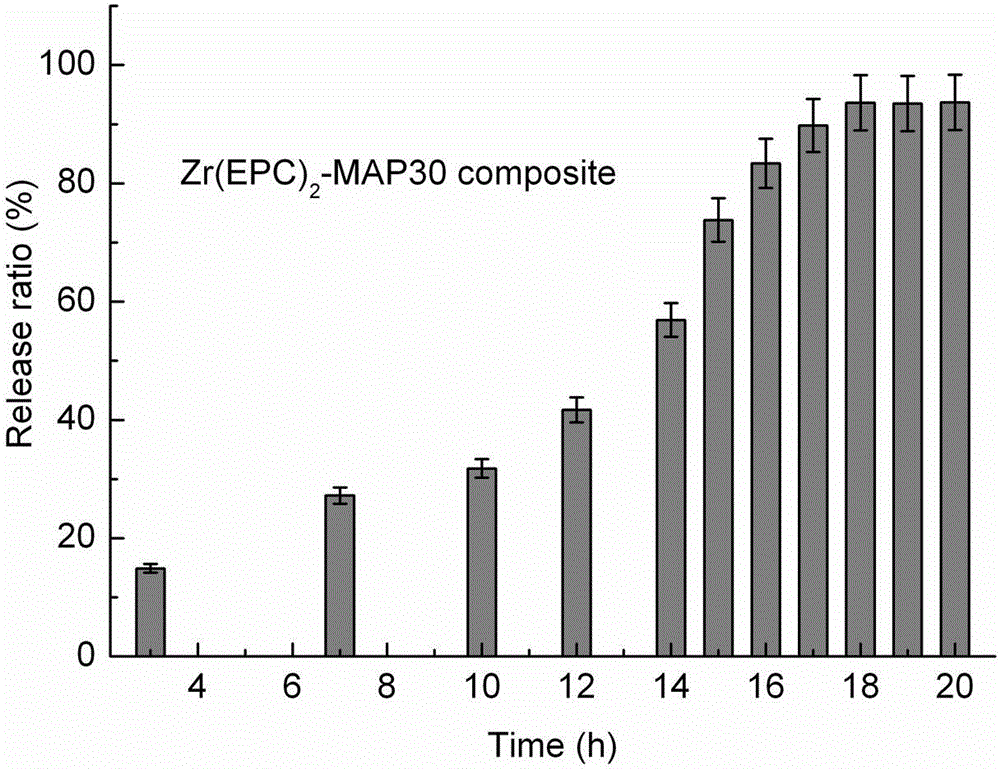
[0047] Zr(EPC) 2 – Drug release from MAP30 liposomes:
[0048] Liposomes were prepared by the following process, protein:carrier (mass ratio) 0.34:1, pH 8.52 (10 mM PBS+100 mM NaCl), reaction time 15 h; liposomes were centrifuged, washed and freeze-dried, wherein MAP30 The content (mass ratio) is 24.0 ± 1.0 %; in 187 μg of liposomes, inject 1.5 ml of fresh in vitro serum samples, stir at 3000 rpm / min for 20 min (4°C), and obtain a translucent sus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com