Preparation method of absorbent used for removing impurities from olefin flows
A technology of adsorbent and olefin flow, which is applied to chemical instruments and methods, adsorption purification/separation, and other chemical processes, and can solve problems such as reduced reactivity, decreased adsorption capacity, increased amount of adsorbent, and increased volume of purification devices. Achieve the effect of low adsorption heat effect and uniform pore distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
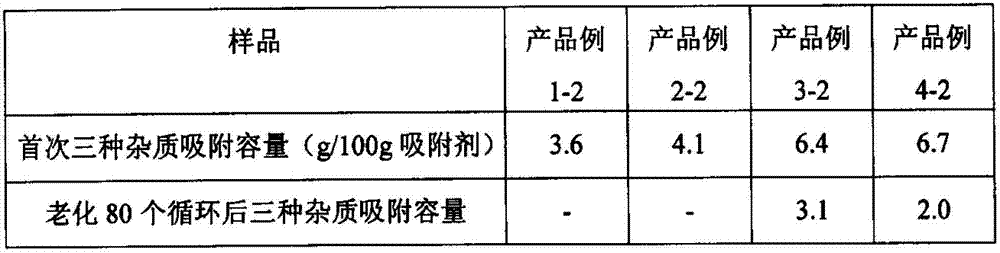
[0032] 24.7g pseudoboehmite (content 63.5%) was mixed with 10g sodium hydroxide and 100g deionized water and stirred for 30 minutes, and 67.1g silica sol (SiO 2 31%, Na 2 O10.2%) mixed with 100g of deionized water and stirred for 20 minutes, slowly added silica sol solution to the container containing aluminum source solution, stirred at high speed for 30 minutes, added 65g of deionized water during the stirring process, and then quickly added 1 ~ 3mm Particle size, specific surface area is 250m 2 / g, 21g of activated alumina balls with a pore volume of 0.35ml / g, stirred for 15 minutes and aged at room temperature 25°C for 4 hours, and the resulting sol containing alumina particles was transferred to a reaction with a polytetrafluoroethylene liner In the kettle, crystallize at 100°C for 12 hours, separate the solid and liquid to take out the product, wash the 1-3mm solid particles with deionized water to pH = 8-9, and dry at 100°C for 10 hours to obtain the intermediate prod...
Embodiment 2
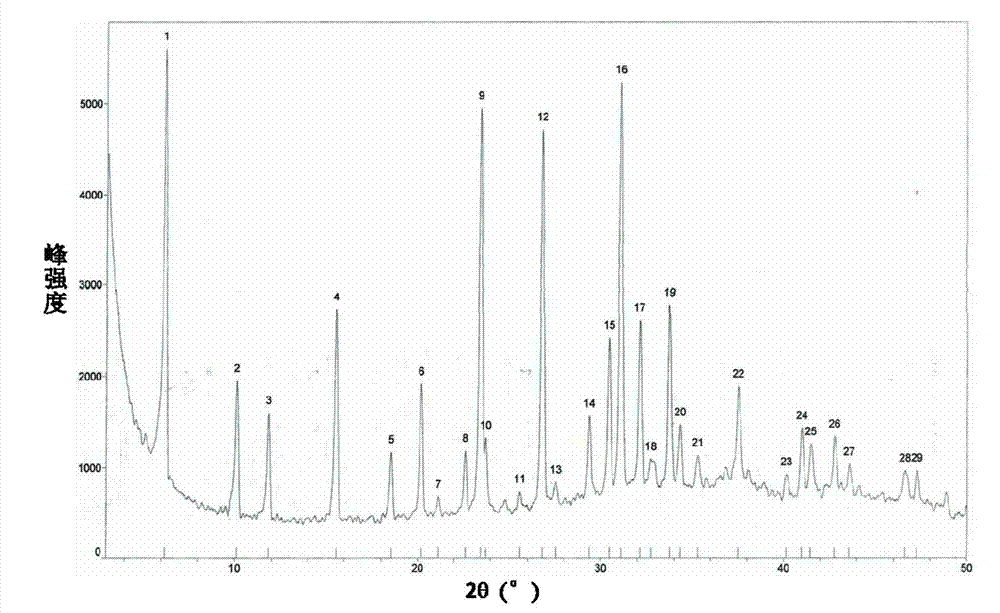
[0034] The molecular sieve sol was prepared according to the above-mentioned Example 1, but the amount of activated alumina spheres was changed from 21 g to 13 g. After stirring for 15 minutes, it was aged at room temperature at 25° C. for 4 hours, and the resulting sol containing alumina particles was transferred into a polysaccharide-containing sol. In a reaction kettle lined with tetrafluoroethylene, crystallize at 100°C for 12 hours, separate the solid and liquid to take out the product, wash the 1-3mm solid particles with deionized water to pH = 8-9, and dry at 100°C for 10 hours to prepare The intermediate product Example 2-1 was obtained, and then activated by calcination at a temperature of 450°C for 2 hours to obtain the product example 2-2. It was confirmed by XRD that the diffraction peak of the molecular sieve was the characteristic peak of 13X. After the oxide composition was tested by XRF, the composition (wt%) of product example 2-2 was 86.6% alumina, 13.4% 13X m...
Embodiment 3
[0036] Using the intermediate product example 2-1 instead of activated alumina balls as the carrier, the rest of the steps are the same as in Example 1, corresponding to the intermediate product example 3-1 and the product example 3-2. It was confirmed by XRD that the diffraction peak of the molecular sieve was the characteristic peak of 13X. After the oxide composition was tested by XRF, the composition (wt%) of product example 3-2 was 78.9% alumina, 21.1% 13X molecular sieve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com