Microwave synthesis method of three asymmetric porphyrins
A microwave synthesis and asymmetric technology, applied in the direction of organic chemistry, can solve the problems of reduced yield, increased by-products, difficult separation and purification of products, etc., to achieve the effects of reduced by-products, high product purity, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
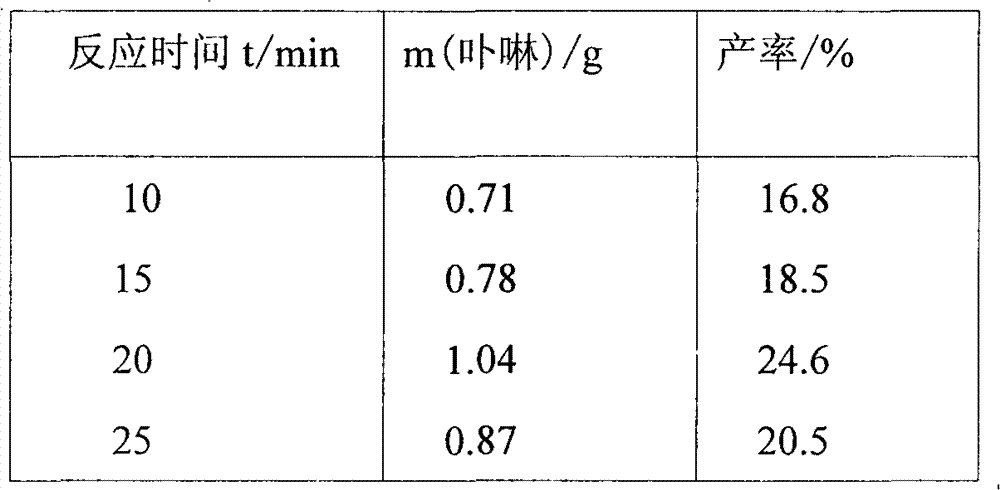
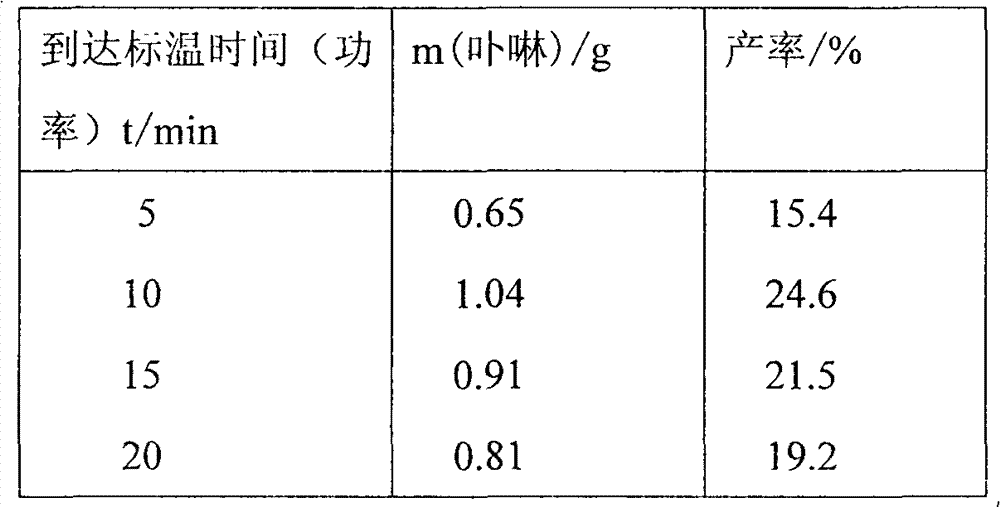
[0026] Weigh 4-hydroxybenzaldehyde (25mmol, 3.05g) in a three-necked flask, measure 60ml of propionic acid to dissolve, place a temperature probe and a condenser tube, fix it in a microwave instrument, set the target temperature to 142°C and the power (to reach the target temperature Time) 10min, start stirring and heating work. Measure a solution of pyrrole (25mmol, 1.75ml) dissolved in 15ml of propionic acid. After reflux, the dropwise addition is completed within 2 minutes, and the microwave reaction is continued for 20 minutes. After the reaction was completed, cool to room temperature, evaporate nearly half of the solvent under reduced pressure, and refrigerate overnight. Suction filtration to obtain a purple-black solid, chloroform was used as eluent, silica gel column chromatography, the first purple-red color band was collected, and vacuum-dried to obtain a blue solid, which was then washed with ethyl acetate-petroleum ether (2:1, V:V) Recrystallization gave 1.04 g of...
Embodiment 2
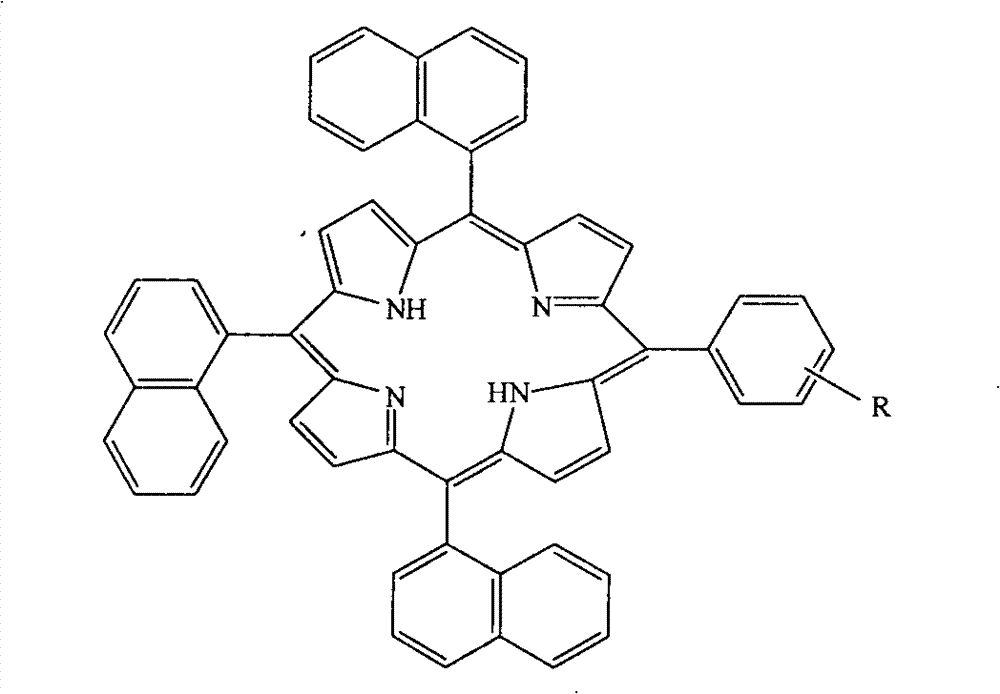
[0028] Dissolve 3.40ml (25mmol) of 1-naphthaldehyde and 1.02g (8.3mmol) of m-hydroxybenzaldehyde in 60ml of propionic acid, heat in microwave to reflux, and add dropwise 2.30ml (33mmol) of pyrrole dissolved in 15ml of propionic acid within 2 minutes. Solution, continue microwave reaction for 20 minutes under the microwave power of 10 minutes. After the reaction was completed, cool to room temperature, evaporate nearly half of the solvent under reduced pressure, add an equal amount of methanol to the remaining mother liquor, and refrigerate overnight. Suction filtration gave a purple-black solid, and the crude product was dichloromethane-petroleum ether (3:1, V: V) Carry out column chromatography as the eluent, collect the purple-red second color band, dry in vacuo to obtain a blue-purple solid, and then recrystallize with ethyl acetate-petroleum ether (2:1, V:V) to obtain a blue-purple crystal, That is, 0.67 g of 5-m-hydroxyphenyl-10,15,20-trinaphthylporphyrin (1), the yield i...
Embodiment 3
[0030] Dissolve 3.40ml (25mmol) of 1-naphthaldehyde and 1.28g (8.3mmol) of 3-hydroxy-4-methoxybenzaldehyde in 60ml of propionic acid, heat to reflux in microwave, and add 2.30ml (33mmol) dropwise within 2 minutes Pyrrole was dissolved in a solution of 15ml propionic acid, and the microwave reaction was continued for 20 minutes under the microwave power of 10 minutes. After the reaction was completed, cool to room temperature, evaporate nearly half of the solvent under reduced pressure, add an equal amount of methanol to the remaining mother liquor, and refrigerate overnight. Suction filtration gave a purple-black solid, and the obtained crude product was dichloromethane-petroleum ether (3:1 , V:V) as the eluent for column chromatography, collecting the purple-red second color band, drying in vacuo to obtain a blue-purple solid, and then recrystallizing with ethyl acetate-petroleum ether (2:1, V:V) to obtain a blue Purple crystal, 5-(3-hydroxy-4-methoxy)phenyl-10,15,20-trinapht...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com