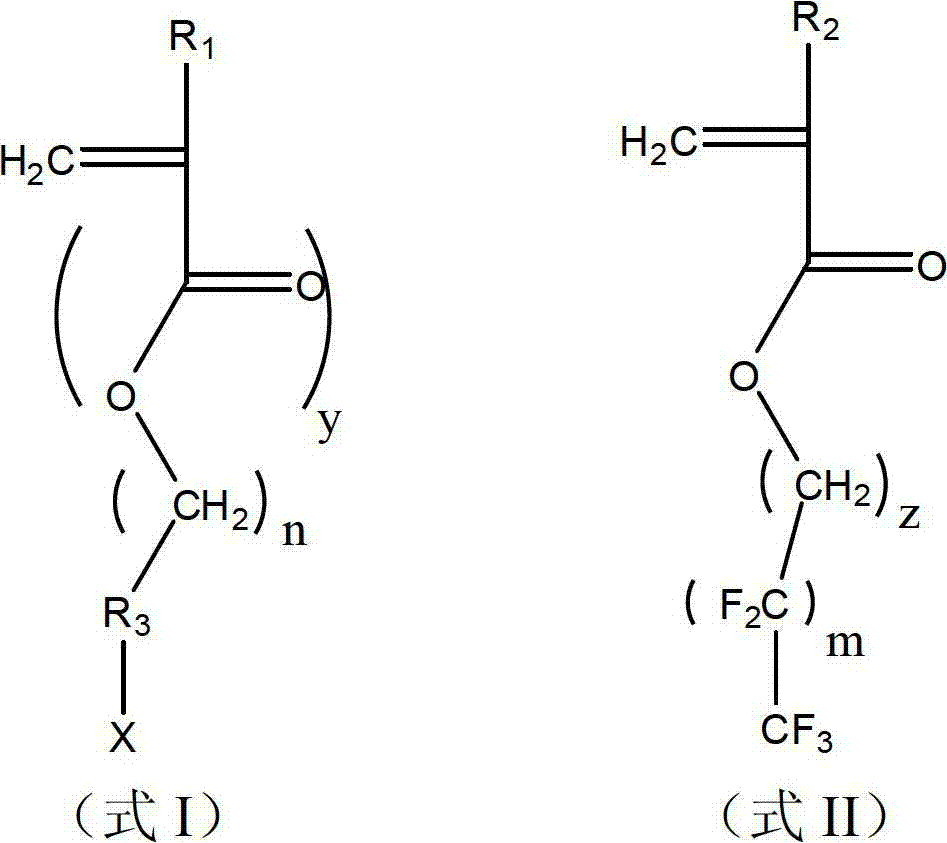Ultraviolet-crosslinking fluorine-containing polymer and application thereof in preparing super-amphiphobic surface
A super-amphiphobic surface and cross-linking technology, which is applied in the direction of manufacturing tools, plant fibers, coatings, etc., can solve the problems of weak adhesion, poor friction resistance and washing resistance, and poor bonding between the super-amphiphobic surface and the substrate. Reliability and other issues, to achieve the effect of simple method, reliable bonding and strong adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesizing UV-crosslinked fluoropolymer by ATRP method comprises the following steps:
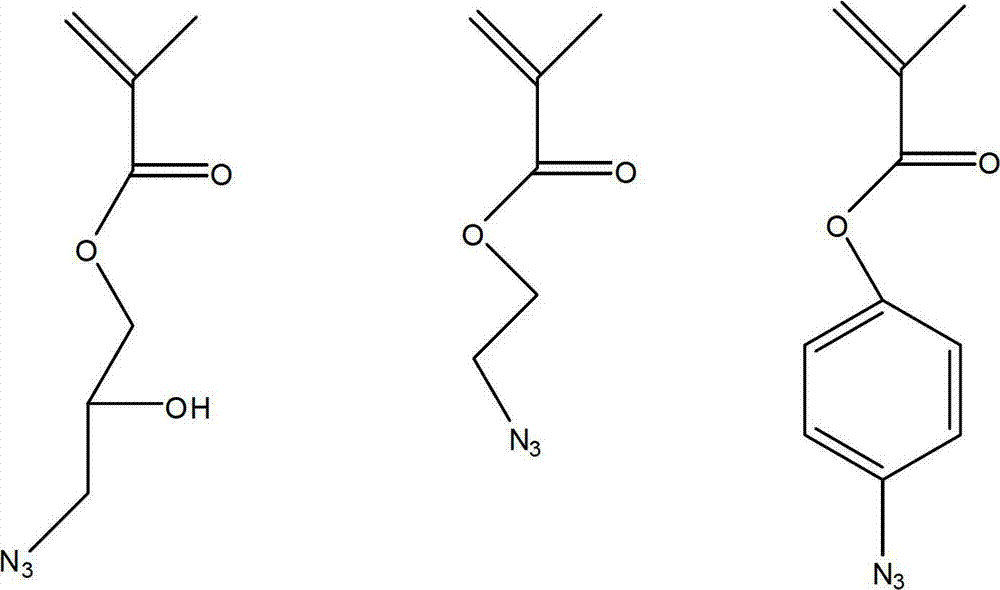
[0042] Add 1.852g of triazide dihydroxypropyl methacrylate (3-azido-2-hydroxypropyl methacrylate, referred to as: AHMA) and 0.203g of 2-bromoisobutyric acid monomethoxyethyl in a 100ml round bottom flask Glycol ester, 0.237g 4,4'-dinonyl-2,2'-bipyridine and 3ml cyclohexanone, stirring and dissolving the reaction system, bubbling with argon gas for 30min, then removing oxygen, and then transferring the reaction system Put 0.1124g of cuprous bromide into a 50ml round-bottomed flask, carry out polymerization reaction at 40°C for 2h, the reaction product is precipitated in methanol, washed with methanol and then washed with n-hexane, and then vacuum-dried at room temperature for 24h to constant weight , to obtain the product polytriazide dihydroxypropyl methacrylate (PAHMA).
[0043] Add 1.5g PAHMA, 1.852g trifluoroethyl methacrylate, 0.737g 4,4'-dinonyl-2,2'-bipyridine and 4ml triflu...
Embodiment 2
[0047] Synthesis of ultraviolet light crosslinking type fluoropolymer by anion polymerization method, comprising the following steps:
[0048] Add 0.19 ml of 1,1-diphenylethylene to a three-neck flask containing 250 ml of anhydrous tetrahydrofuran at -78°C (dry ice acetone bath), followed by adding 0.6 ml of 1.4 mol / L sec-butyllithium in hexane solution. After 25 minutes, 25.19 ml of azidoethyl methacrylate (2-azidoethyl methacrylate, referred to as: AMA) was added, 1.24 ml of pentafluoroethyl methacrylate was added after the polymerization reaction was carried out for 1 hour, and the polymerization reaction was continued for another 2 hours before adding 1.0 mL of anhydrous methanol was used to terminate the polymerization. After the reaction system was warmed up to 23° C., it was concentrated by distillation to 100 ml, and then the polymer was precipitated in excess methanol, filtered and dried in a vacuum oven to obtain the desired polymer PAMA-b-PFEMA.
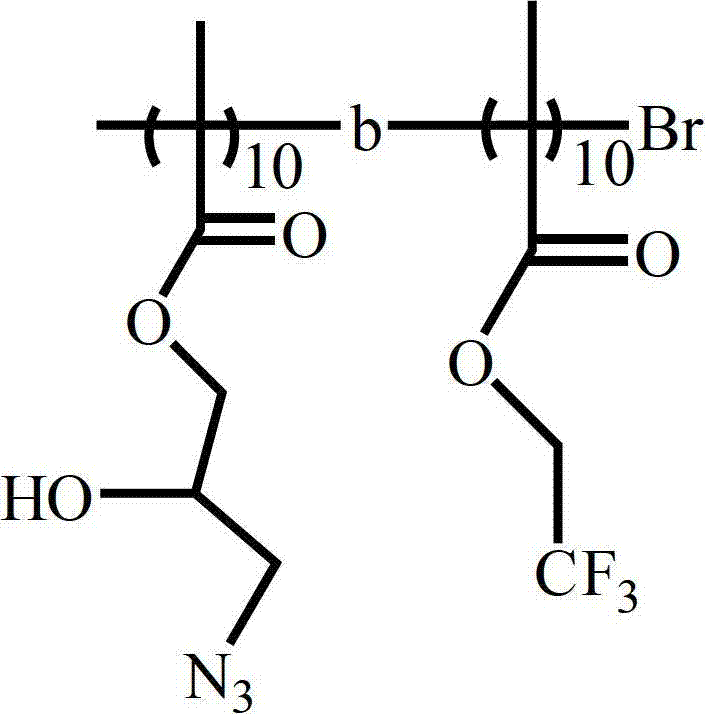
[0049] The struc...
Embodiment 3
[0052] The free radical method synthesizes the ultraviolet light cross-linking type fluoropolymer, comprises the following steps:
[0053] Add 15g perfluorooctyl ethyl acrylate, 1.852g azidophenyl methacrylate (4-azidophenyl methacrylate, ADMA for short), 0.174g AIBN as initiator and 50ml trifluorotoluene into a 100ml round bottom flask, The reaction system was stirred and dissolved, bubbled with argon for 30 minutes, and polymerized at 90°C for 8 hours. The reaction product was precipitated in methanol, washed with methanol and then washed with n-hexane, and then vacuum-dried at 40°C for 24 hours to constant weight to obtain product.
[0054] The structure of the product is shown below:
[0055]
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com